User:Gastro guy/bhom work
| Gastro guy/bhom work |
|---|
Blastocystis is a highly prevalent single-celled parasite that infects the gastrointestinal tract of humans and animals. Many different types of Blastocystis exist, and the organism is able to infect humans, farm animals, birds, rodents, cockroaches, amphibians, reptiles, and fish. Blastocystis has presented a challenge to the medical and scientific community due to the diversity of hosts the organism can infect, the large number of different species, and the fact that most of them seem to be able to cause infection in humans. Blastocystis was originally classified as a yeast, then a protozoan. Genetic analysis has placed it in the Stramenopile kingdom.
Symptoms
[edit]Symptoms in animals: Experimental infection in immunocompetent and immunocompromised mice has produced death, intestinal inflammation, invasive infection and lethargy. [1] [2] [3] Chronic diarrhea has been reported in non-human higher primates. [4]
Symptoms in humans: Researchers have published conflicting reports concerning whether Blastocystis causes symptoms in humans, with one of the earliest reports in 1916. [5] The incidence of reports associated with symptoms began to increase in 1984 [6], with physicians from overseas reporting symptoms in humans [7] and US physicians reporting symptoms in individuals with travel to less developed countries. [8] A lively debate ensued in the early 1990's, with some physicians objecting to publication of reports that Blastocystis caused disease [9][10][11][12] Some researchers believe the debate has been resolved by finding of multiple species of Blastocystis that can infect humans, with some causing symptoms and others being harmless (see Genetics and Symptoms).
The most commonly reported symptoms are abdominal pain constipation, diarrhea, weight loss, fatigue, and flatulence. Less commonly reported symptoms are:
- Intestinal inflammation [13]
The following table describes studies of patients infected with Blastocystis where that was the only causing organism found in stool samples:
| Patient Studies of Symptoms Associated with Blastocystis Infection | |||
|---|---|---|---|
| Year | #
of Patients |
Location | Symptoms |
| 1989 | 19 | Albert Einstein College of Medicine | Abdominal pain, diarrhea, weight loss, flatulence [8] |
| 1990 | -- | Lackland Air Force Base | Abdominal pain, weight loss, nausea, mild diarrhea [21] |
| 1990 | -- | Metro-McNair Clinic | Diarrhea, abdominal pain, flatulence [22] |
| 1991 | 239 | King Faisal hospital | Abdominal pain (87.9%), constipation (32.2%), diarrhea (23.4%), headache, fatigue, depression [7]. |
| 1991 | 31 | Farwaniya Hospital | Abdominal pain, diarrhea, flatulence, weight loss [23] |
| 1992 | 39 | University of Pittsburgh School of Medicine | Abdominal pain, diarrhea, vomiting, weight loss [24] |
| 1994 | 175 | Jordan | Recurrent mild diarrhea, nausea, abdominal pain, weight loss. [25] |
| 2005 | 106 | Egypt | Diarrhea (30.4%), abdominal pain (26.1%), flatulence (21.7%), vomiting (13.1%) fatigue (8.7%). [26] |
| 2006 | 824 | United States | Diarrhea, bloating, flatulence. Also fatigue, fever/headeache, dermatitis. [15] |
| 2006 | 89 | Turkey | Stunted growth [27] |
Treatment
[edit]Despite its prevalence, there is a lack of study regarding treatment of the infection. In-vitro antibiotic sensitivity testing is rarely reported. The following first-line treatments have been reported as successful in studies:
- Metronidazole was reported as 100% successful in a 1991 study [7] however in a subsequent study it was found to be less effective especially in patients classified as severely infected. [28]
- TMP-SMX was reported to slightly less effective than Metronidazole in one study.
- Nitazoxanide was reported to be successful in a study authored by the drug's manufacturer.[29]
- Iodoquinol and Paromomycin have been reported in the treatment of Blastocystis infection. [30] Iodoquinol has been found to be less effective in practice and in-vitro. [31] [32]
- Miconazole has been reported as an agent against Blastocystis growth in-vitro. [33]
Treatment Failure: A 1916 report of Blastocystis infection described it as "an infection that is hard to get rid of," [5] suggesting treatment failure may not be a recent phenomenon. A clinical report from 1986 identified cases that were non-responsive to several different antiprotozoal drugs. [34] An in-vitro study found 40% of isolates are resistant to common antiprotozoal drugs. [35] A study of isolates from patients diagnosed with IBS found 40% of isolates resistant to Metronidazole and 32% resistant to furazolidone. [36] The problem of refractory cases is also described in a review article from an NIH lab [6] and in a US report [37]
Treatments that are not readily available
[edit]Physicians have described the successful use of a variety of discontinued antiprotozoals in treatment of Blastocystis infection. The reduction in the availability of antiprotozoal drugs has been noted as a complicating factor in treatment of other protozoal infections. [38] For example, in Australia, production of diloxanide furoate ended in 2003, paromomycin is available under special access provisions, and the availability of iodoquinol is limited. [39] The following discontinued drugs have been reported as effective in treating Blastocystis infection:
- Emetine: Use was reported as successful in cases in early 20th century with British soldiers who contracted Blastocystis infection while serving in Egypt. [5] In-vitro testing showed emetine was more effective than Metronidazole or furazolidone. [40] Emetine is available in the United States only through special arrangement with the Center for Disease Control.
- Clioquinol (Entero-vioform): An NIH researcher noted that this drug was successfull in treatment of Blastocystis infection but removed from the market following an adverse event in Japan. [6]
- Stovarsol, Carsavol, and Narsenol: These arsenic-based antiprotozoals were used extensively for treatment of bacterial and protozoal infections well into the 20th century. They have been reported to be effective against the infection. [6] They are no longer available for therapeutic use. In the United States, other arsenic-based antiprotozoals are still used in the treatment of Leishmania infection through special arrangement with the Center for Disease Control
Diagnostic methods that are clinically available
[edit]Diagnosis is performed by determining if the infection is present, and then making a decision as to whether the infection is responsible for the symptoms. Diagnostic methods in clinical use have been reported to be of poor quality and more reliable methods have been reported in research papers. [41][42] [43] [44] [45]
For identification of infection, the only method clinically available in most areas is the Ova and Parasite exam, which identifies the presence of the organism by microscopic examination of a chemically preserved stool specimen. This method is called "Direct Microscopy". In the United States, pathologists are required to report the presence of Blastocystis, so a special test does not have to be ordered. Direct Microscopy is inexpensive, as the same test can identify a variety of gastrointestinal infections, such as Giardia, Entamoeba histolytica, Cryptosporidium. However one laboratory director noted that pathologists using conventional microscopes failed to identify many infections, and indicated the use of special equipment for identification. [12] The following table shows the sensitivity of direct microscopy in various studies when compared to stool culture. A recent study has suggested that stool culture can identify only 83% of individuals infected (when compared to PCR testing). [44]
| Sensitivity
(% Samples |
Year of Study | Geographic Location | Method | # Samples
identified |
|---|---|---|---|---|
| 19% | 2002 | Thailand | Direct Microscopy with concentration | (64/334) [42] |
| 43% | 2002 | Thailand | Direct Microscopy of stool smear | (134/334) [42] |
| 0% | 2004 | Scotland | Formol-Ether Concentration | (0/39) [43] |
| 16.7% | 2004 | Thailand | Stool Smear | [46] |
| 40.7% | 2004 | Thailand | Trichrome stain | |
| 66% | 2006 | Denmark | Formol-Ether Concentration | (compared to PCR) [44] |
Reasons given for the failure of Direct Microscopy include: (1) Variable Shedding: The quantity of Blastocystis organisms varies substantially from day to day in infected humans and animals; [47] (2) Appearance: Some forms of Blastocystis resemble fat cells or white blood cells [44], making it difficult to distinguish the organism from other cells in the stool sample; (3) Large number of morphological forms: Blastocystis cells can assume a variety of shapes, some have been described in detail only recently, so it is possbile that additional forms exist but have not been identified. [44]
Several methods have been cited in literature for determination of the significance of the finding of Blastocystis:
- Diagnosis only when large numbers of organism present: Some physicians consider Blastocystis infection to be a cause of illness only when large numbers are found in stool samples. [26] Researchers have questioned this approach, noting that it is not used with any other protozoal infections, such as Giardia or Entamoeba histolytica. Some researchers have reported no correlation between number of organisms present in stool samples and the level of symptoms. [22] A study using PCR testing of stool samples suggested that symptomatic infection can exist even when sufficient quantities of the organism do not exist for identification through Direct Microscopy. [44]
- Diagnosis-by-exclusion: Some physicians diagnose Blastocystis infection by excluding all other causes, such as infection with other organisms, food intolerances, colon cancer, etc. This method can be time consuming and expensive, requiring many tests such as endoscopy and colonoscopy.
- Disregarding Blastocystis : In the early to mid 1990's, some US physicians suggested all findings of Blastocystis are insignificant. No recent publications expressing this opinion could be found. [9] [48]
Diagnostic methods that are not clinically available
[edit]The following diagnostic methods are not routinely available to patients. They have been used in research labs and reportedly are more reliable at detecting infection and are able to distinguish between symptomatic and asymptomatic infection. That is, they are reportedly able to provide the physician with information as to whether Blastocystis infection is the cause of the patient's symptoms:
Serum antibody testing: A 1993 research study performed by the NIH with United States patients suggested that it was possible to distinguish symptomatic and asymptomatic infection with Blastocystis using serum antibody testing. [49] The study used blood samples to measure the patient's immune reaction to chemicals present on the surface of the Blastocystis cell. It found that patients diagnosed with symptomatic Blastocystis infection exhibited a much higher immune response than controls who had Blastocystis infection but no symptoms. The study was repeated in 2003 at Ain Shams University in Egypt with Egyptian patients with equivalent results .
Fecal Antibody Testing: A 2003 study at Ain Shams University in Egypt indicated that patients symptomatically infected could be distinguished with a fecal antibody test. The study compared patients diagnosed with symptomatic Blastocystis infection to controls who had Blastocystis infection but no symptoms. In the group with symptoms, IgA antibodies to Blastocystis were detected in fecal specimens that were not present in the healthy control group.
Stool Culture: Culturing has been shown to be a more reliable method of identifying infection. In 2006, researchers reported the ability to distinguish between disease causing and non-disease causing isolates of Blastocystis using stool culture. [50] Blastocystis cultured from patients who were sick and diagnosed with Blastocystis infection produced large, highly adhesive amoeboid forms in culture. These cells were absent in Blastocystis cultures from healthy controls. Subsequent genetic analysis showed the Blastocystis from healthy controls was genetically distinct from that found in patients with symptoms. Protozoal culture is unavailable in most countries due to the cost and lack of trained staff able to perform protozoal culture.
Genetic Analysis of isolates: Researchers have used techniques which allow the DNA of Blastocystis to be isolated from fecal specimens. [51] [45] [44] This method has been reported to be more sensitive than stool culture in symptomatic patients. This method also allows the species group of Blastocystis to be identified. Research is continuing into which species groups are associated with symptomatic (see Genetics and Symptoms)
Immuno-Fluorescence (IFA) Stain: An IFA stain causes Blastocystis cells to glow when viewed under a microscope, making the diagnostic method more reliable. IFA stains are in use for Giardia and Cryptosporidium for both diagnostic purposes and water quality testing. A 1991 paper from the NIH described the laboratory development of one such stain.[6] However, no company currently offers this stain commercially.
Transmission and risk factors
[edit]Humans contract Blastocystis infection by drinking water or eating food contaminated with feces from an infected human or animal. [52] Blastocystis infection can be spread from animals to humans, from humans to other humans, from humans to animals, and from animals to animals.[53] [45] Risk factors for infection have been reported as following:
- International travel: Travel to less developed countries has been cited in development of symptomatic Blastocystis infection.[54] A 1986 study in the United States found that all individuals symptomatically infected with Blastocystis reported recent travel history to less developed countries. [8] In the same study, all hospital employees working in New York who were screened for Blastocystis were found to have asymptomatic infections.
- Military service: Several studies have identified high rates of infection in military personnel. An early account described infection of British troops in Egypt in 1916 [5] who recovered following treatment with emetine. A 1990 study published in Military Medicine from Lackland AFB in Texas concluded symptomatic infection was more common in foreign nationals, children, and immunocompromised individuals. [21] A 2002 study published in Military Medicine of army personnel in Thailand identified a 44% infection rate. Infection rates were highest in privates who had served the longest at the army base. [55] A follow-up study found a significant correlation between infection and symptoms, and identified the most likely cause as contaminated water. [55] A 2007 newspaper article suggested the infection rate of US military personnel returning from the Gulf War was 50%, quoting the head of Oregon State University's Biomedicine department. [56] An 2007 article published in Medical Hypotheses magazine noted that the symptoms reported in medical literature for Blastocystis were similar to those reported by returning Persian Gulf War veterans. [57]
- Consumption of Untreated Water (well water): Many studies have linked Blastocystis infection with contaminated drinking water. A 1993 study of children infected symptomatically with Blastocystis in Pittsburgh indicated that 75% of them had a history of drinking well water or travel in less developed countries. Two studies in Thailand linked Blastocystis infection in military personnel and families to drinking of unboiled and untreated water. [58] [55] A book published in 2006 noted that in an Oregon community, infections are more common in winter months during heavy rains. [37]. A research study published in 1980 reported bacterial contamination of well water in the same community during heavy rainfall. [59] A 2007 study from China specifically linked infection with Blastocystis sp. subtype 3 with drinking untreated water. [60] Recreational contact with untreated water, for example though boating, has also been identified as a risk factor [37]. Studies have shown that Blastocystis survives sewage treatment plants in both the United Kingdom and Malaysia. [61] Blastocystis cysts have been shown to be resistant to chlorination as a treatment method [62] and are among the most resistant cysts to ozone treatment. [63]
- Contaminated Food: Contamination of leafy vegetables has been implicated as a potential source for transmission of Blastocystis infection, as well as other gastrointestinal protozoa. [64] A Chinese study identified infection with Blastocystis sp. subtype 1 as specifically associated with eating foods grown in untreated water. [60]
- Daycare usage: A Canadian study identified an outbreak of Blastocystis associated with daycare usage. [65] Prior studies have identified outbreaks of similar protozoal infections in daycares. [66]
- Geography: Infection rates vary geographically, and variants which produce symptomatic infection may be less common in developing countries. For example, a low incidence of Blastocystis infection has been reported in Japan. And a study of individuals infected with Blastocystis in Japan found that most (43%, 23/54) carried Blastocystis sp. subtype 2, which was found to produce no symptoms in 93% (21/23) of patients studied. Studies in urban areas of industrialized countries have found Blastocystis infection associated with a low incidence of symptoms. [67] In contrast, studies in developing countries generally show Blastocystis as being associated with symptoms [68][7]. A higher incidence of Blastocystis infection has been reported in California and West Coast states. [69] [15]
- Time: A 1989 study of the prevalence of Blastocystis in the United States found an infection rate of 2.6% in samples submitted from all 48 states. [69] The study was part of the CDC's MMWR Report. A more recent study in 2006 found an infection rate of 23% samples submitted from all 48 states. The more recent study was performed by a private laboratory located in the Western US, and emphasized samples from Western states which have previously been reported to have a higher infection rate. [69]
Research studies have suggested the following items are not risk factors for contracting Blastocystis infection:
- Consumption of municipal water near water plant not a risk factor): One study showed that municipal water was free of Blastocystis even when drawn from a polluted source. However, samples taken far away from the treatment plant showed cysts. The researchers suggested that aging pipes may permit intrusion of contaminated water into the distribution system.[70]
- Human-to-Human transmission among adults (not a risk factor): Some research suggests that direct human-to-human transmission is less common even in households and between married partners. One study showed different members of the same household carried different subtypes of Blastocystis. [51]
Prevalence
[edit]Like other protozoal infections, the prevalence of Blastocystis infection varies depending on the area investigated and the population selected. A number of different species groups of Blastocystis infect humans [71], with some being reported to cause disease while others do not. [51] [72] To date surveys have not distinguished between different types of Blastocystis in humans so the significance of findings may be difficult to evaluate. Developing countries have been reported to have higher incidences, however recent studies suggest that symptomatic infection with Blastocystis may be prevalent in certain areas of industrialized countries as well:
- A nation-wide study conducted by the CDC using data reported from 1987 found the prevalence Blastocystis infection in the United States to be 2.6%.[69] The study indicated that Western states, such as California, reported a higher prevalence.
- A 2000 study by a private laboratory of stool samples from 48 states in the United States identified a prevalence of 23%. [73] The study was conducted by a laboratory in Arizona and emphasized Western states which have previously been found to have higher rates of Blastocystis infection.
- A Canadian study of samples received in 2005 identified Blastocystis as the most prevalent protozoal infection identified. [74]
- A study in Pakistan identified Blastocystis infection in 7% of the general population and 46% of patients with irritable bowel syndrome. The study used stool culture for identification.
Phylogenetics and symptoms
[edit]Physicians have produced conflicting reports regarding whether Blastocystis causes disease in humans. These reports resulted in a brief debate in medical journals in the early 1990's between some physicians in the United States who believed that Blastocystis was harmless, and physicians in the United States and overseas who believed it could cause disease.
At the time, it was common practice to identify all Blastocystis from humans as Blastocystis hominis, while Blastocystis from animals was identified differently (i.e. Blastocystis ratti from rats). Genetic research performed since then has shown that genetically speaking, no organism known as Blastocystis hominis exists. Rather, humans can acquire infection from any one of nine species groups of Blastocystis which are also carried by cattle, pigs, rodents, chickens, pheasants, monkeys and other animals. [71] [53]. Research has suggested that some types produce few or no symptoms, while others producing illness and intestinal inflammation. [72] [51] Researchers have suggested conflicting reports may be due to the practice of naming all Blastocystis from humans Blastocystis hominis. [53] and have proposed discontinuing the use of the term Blastocystis hominis to avoid further confusion.[53]
A standard naming system for Blastocystis organisms from humans and animals has been proposed which names Blastocystis isolates according to the genetic identity of the Blastocystis organism rather than the host. [53] The naming system used identifies all isolates as Blastocystis sp. subtype nn where nn is a number from 1 to 9 indicating the species group of the Blastocystis organism. The identification of the species can not be performed with a microscope at this time, because the different species look alike. Identification requires equipment for genetic analysis that is common in microbiology laboratories, but not available to most physicians. Some new scientific papers have begun using the standard naming system. [75]
Because of the large number of species and conflicting reports, researchers have focused on the possibility that different species cause different degrees of illness.
- A German researcher, Dr. Kukoschke, was one of the first researchers to suggest that different species of Blastocystis were present in symptomatic and asymptomatic individuals. In a 1992 study, he noted that isolates from sick patients with Blastocystis would not grow in human serum, but rather required rabbit or horse serum to grow. [76] Isolates from symptomatic individuals would not grow well in human serum, but required horse serum instead.
- In 1993, Dr. Charles Zierdt, an NIH researcher, used a serum antibody test in study of individuals infected with Blastocystis. He compared the results from subjects with symptoms to those who were healthy. [49] His 1993 publication showed that the subjects with symptoms had much higher levels of antibodies to Blastocystis in blood samples, and that a dividing line could be drawn separating the two groups. At this time a similar finding had been made relative to Entamoeba histolytica, and researchers were developing methods to distinguish between the pathogenic Entamoeba histolytica species, and the harmless Entamoeba dispar species. In his paper, he drew a parallel to the research into that protozoan.
- A 1994 study Dr. DW MacPherson of St. Joseph's Hospital in Canada noted variation in cyst forms and suggested different species were present. [77]
- A 1999 study by Dr. M Lanuza, et al of the University of Valencia, Spain found that the protein profiles exhibited in Blastocystis cultures from symptomatic patients differed from those of patients without symptoms. [78]
- The first genetic study differentiating symptoms was published in 2003 by Japanese researcher, Dr. Yoshimasa Kaneda. [51] This study reported that the most common species group in Japan was Blastocystis sp. subtype 2 and it caused no symptoms in 93% (21/23) of individuals examined. Species groups Blastocystis sp. subtype 1,3, and 6 were found to cause symptoms in about half of individuals studied. These species groups are carried by farm animals and birds. [71]
- In 2003, Zierdt's serum antibody research results were repeated by Dr. Mahmoud at Aga Khan University in Egypt.
- In 2006, researchers at the University of Malaysia reported that isolates from symptomatic individuals produced large amoeboid forms when cultured in the presence of bacteria. These forms were absent from isolates cultured from healthy carriers. The study noted a 100% association which had not been found previously, and suggested that previous studies had not cultured the organisms long enough, as at least 4 days were required for some cultures to produce amoeboid forms. The group also performed genetic analysis of isolates from symptomatic individuals and found they belonged to a narrow genetic group, while isolates from asymptomatic carriers were found to be from a genetically diverse clade. [72]
- A 2006 study performed by Dr. Rune Stensvold with patients being diagnosed in Denmark identified Blastocystis sp. subtypes 3 and 4 in most patients with diarrhea in whom no other cause could be found. [44]
Pathogeneses (how it causes disease)
[edit]Pathogenesis refers to the mechanism by which an organism causes disease. The following disease-causing mechanisms have been reported in studies of Blastocystis infection:
- Barrier Disruption: In isolates from Blastocystis sp. subtype 4, study has also demonstrated that Blastocystis has the ability to alter the arrangement of F-actin in intestinal epithelial cells. Actin filaments are important in stabilizing tight junctions; they in turn stabilize the barrier, which is a layer of cells, between the intestinal epithelial cells and the intestinal content. [79] The parasite causes the actin filaments to rearrange, and so compromising barrier function. This has been suggested to contribute to the diarrheal symptoms sometimes observed in Blastocystis patients.
- Immune Modulation: Blastocystis has been shown to provoke cells from the human colon to produce inflammatory cytokines Interleukin-8 and GM-CSF. [81] Interleukin-8 plays a role in rheumatoid arthritis.
- Protease Secretion: Blastocystis secretes a protease that breaks up antibodies produced and secreted into the gastrointestinal tract lumen. [82] These antibodies, known as immunoglobulin A (IgA), make up the immune defense system of human by preventing the growth of harmful microorganisms in the body and by neutralizing toxins secreted by these microorganisms. By breaking up the antibodies, it allows the persistence of Blastocystis in the human gut. Another more recent study has also shown and proposed that, in response to the proteases secreted by Blastocystis, the intestinal host cells would signal a series of events to be carried out, eventually leading to the self-destruction of the host cells – a phenomenon known as apoptosis [79]
- Other secretory mechanism: A study of Entamoeba histolytica found that organism secretes several neurologically active chemicals, such as serotonin and Substance P. [83] [84] Serum levels of serotonin have been found to be elevated in patients with Entamoeba histolytica. [85] One paper noted the diffuse symptoms of Blastocystis infection correllate well with serotonin receptors in the human body, and suggested this may be a mechanism in Blastocystis as well. [57]
Association with irritable bowel syndrome (IBS) and inflammatory bowel disease
[edit]The following reports have linked Blastocystis infection to Irritable bowel syndrome:
- A study of IBS patients in the Middle East found 43% were infected with Blastocystis vs. 7% of healthy controls. [36]
- An additional study of IBS patients in the Middle East showed a "significantly increased" immune reaction in IBS patients to Blastocystis, even when the organism could not be identified in stool samples. [86]
- A European study compared Blastocystis infection rates in IBS patients to those of healthy controls and found a statistically significant infection rate in IBS patients. [87]
- Early reports from the US physicians in the 1980's suggested the presence of the organism was not relevant to the diagnostic process, and patients infected with Blastocystis could be diagnosed with IBS. [88]
The following reports have linked Blastocystis infection to inflammatory bowel disease:
- A study which controlled for Blastocystis species identified Blastocystis sp. subtypes 3 and 6 as the two subtypes which produce intestinal inflammation. [51]
- A case report described inflammatory bowel disease in conjunction with Blastocystis infection. [13]
- Three research groups have reported experimental infection of mice with Blastocystis produces intestinal inflammation. [3] [1] [2]
- An article in a non-peer reviewed medical journal noted that the increase in Blastocystis case reports coincided with with reported increases in the prevalence of inflammatory bowel disease from several European countries [57].
Microbiology
[edit]The appropriate classification of Blastocystis has only recently been resolved. The original description of Blastocystis by it as a yeast due to its yeast-like glistening appearance in fresh wet mounts and the absence of pseudopodia and locomotion. [89] This was then contradicted by Zierdt who reclassified it under subphylum Sporozoa based on some distinctive protistan features that the Blastocystis cell has, such as the presence of nuclei, smooth and rough endoplasmic reticulum, Golgi complex, and mitochondrion-like organelles. Its sensitivity to antiprotozoal drugs and its inability to grow on fungal media further indicated that it was a protozoan. However, some revisions were made to the classification system recently based on modern molecular approaches to classification and studies have shown that Blastocystis is neither yeast nor a protozoan. It is placed in a new Kingdom known as the Stramenopiles. Other Stramenopiles include red agae, the organism that caused the Irish potato famine, and the organism responsible for Sudden oak death disease.
The great diversity of forms in which Blastocystis exists in poses identification and diagnostic problems. Four commonly described forms are the vacuolar (otherwise known as central body), granular, amoeboid, and cyst forms. The appearance of the organism is largely dependent upon environmental conditions as it is extremely sensitive to oxygen.
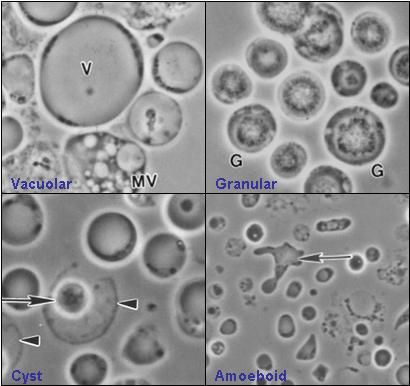
- Vacuolar form
- The vacuolar form is the typical cell form of Blastocystis and is often used for the identification of the organism. These vacuolar forms vary greatly in size, with diameters ranging between 2 µm and 200µm. The vacuolar form is otherwise known as central body form because it has a large central vacuole surrounded by a thin band of peripheral cytoplasm which contains other organelles. Flocculent material has been described as being scattered unevenly throughout the vacuole. The function of the vacuole is still unclear, however, it has been suggested that, like for many eukaryotic cells, it is for storage purposes. Other functions, such as cell division during reproduction and the deposition of apoptotic bodies, have been proposed, although more tests need to be done to validate these roles.
- Granular form
- The granular form is somewhat morphologically identical to the vacuolar forms except that distinct granules were observed in the central vacuole and / or cytoplasm. Within the central vacuole, these granules appear in different forms too. Three types were suggested – metabolic, lipid, and reproductive granules. Metabolic granules play a role in chemical processes that are necessary for the maintenance of life in the organism. It was also put forward that reproductive granules were involved in the development of progeny cells. These hypothesis were made based on microscopy, which may be deemed misleading because of its existence of various cell types, hence more need to be done before making a definite conclusion. It has also been suggested that the granules may be an indication that the cell is dying!
- Amoeboid form
- The other form that exists is the amoeboid form. The amoeboid form of Blastocystis is non-motile and strongly adhesive. A research study has reported that amoeboid forms are produced only in cultures taken from symptomatic individuals, with asymptomatic individuals producing exclusively vacuolar forms. The study suggested this method could be used for diagnosing symptomatic infection. Additionally, it suggested the symptoms could be due to the accumulation of the strongly adhesive amoeboid forms on the host's intestinal wall. A detailed ultra-structural study of amoeboid forms was published in 2007. [50]
- Cyst form
- The Blastocystis cyst form is a more recent discovery and has helped in the advancement of understanding the way it transmits disease. As compared to the other forms, it is generally smaller in size and has a thick multilayered cyst wall. It lacks a central vacuole but few nuclei, multiple vacuoles and food storage deposits were observed. The cyst form is the most resistant form of this parasite and is able to survive in harsh conditions because of its thick multilayered cyst wall. Experiments have been carried out to show its ability to withstand acidic gastric juices. Besides, the cysts did not lyse when placed in distilled water and could survive well at room temperature for up to 19 days, indicating its strong resistance. [90][91] In another experiment, the cyst form was even able to survive in culture medium containing antiprotozoal drugs! This further supports the idea that the cyst form is the most resistant of the four forms.
The proposed life cycle begins with ingestion of the cyst form. After ingestion, the cyst develops into other forms which may in turn re-develop into cyst forms. Through human feces, the cyst forms enter the external environment and are being transmitted to human and animals via the fecal-oral route, repeating the entire cycle.
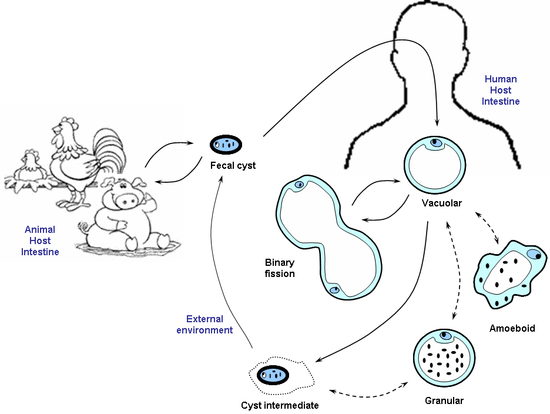
Obtaining and culturing Blastocystis
The ATCC maintains a collection of Blastocystis isolates. Some records show whether the isolates were obtained from symptomatic or asymptomatic carriers. As yet, no publication has identified the subtypes of the isolates. These isolates are axenicized. Researchers have reported that patients with Irritable bowel syndrome may provide a reliable source for xenic Blastocystis isolates. Some researchers have reported being able to culture Blastocystis from 46% of IBS patients. [36] Researchers have described different culture mechanisms for growing Blastocystis. Colony growth on solid medium colonies on solid culture medium using a synthetic medium with added supplements have both been described. [92][93]
References
[edit]- ^ a b Yao FR, Qiao JY, Zhao Y, Zhang X, Yang JH, Li XQ (2005). "[Experimental infection of mice with Blastocystis hominis]". Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi (in Chinese). 23 (6): 444–8. PMID 16566218.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Moe KT, Singh M, Howe J; et al. (1997). "Experimental Blastocystis hominis infection in laboratory mice". Parasitol. Res. 83 (4): 319–25. PMID 9134552.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b c Zhang HW, Li W, Yan QY, He LJ, Su YP (2006). "[Impact of blastocystis hominis infection on ultrastructure of intestinal mucosa in mice]". Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi (in Chinese). 24 (3): 187–91. PMID 17094618.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ McClure, H. M., E. A. Strobert (1980). "Blastocystis hominis in a pig-tailed macaque: a potential enteric pathogen for non-human primates". Lab. Anim. Sci. 30: 890–899. PMID 7191935.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Low GC. "Two chronic amoebic dysentery carriers treated by emetine, with some remarks on the treatment of Lamblia, Blastocystis and E. coli infections". J. Trop. Med. Hyg. (19): 29–34.
- ^ a b c d e Zierdt CH (1991). "Blastocystis hominis--past and future". Clin. Microbiol. Rev. 4 (1): 61–79. PMID 2004348.
- ^ a b c d e f Qadri SM, al-Okaili GA, al-Dayel F (1989). "Clinical significance of Blastocystis hominis". J. Clin. Microbiol. 27 (11): 2407–9. PMID 2808664.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Sheehan DJ, Raucher BG, McKitrick JC (1986). "Association of Blastocystis hominis with signs and symptoms of human disease". J. Clin. Microbiol. 24 (4): 548–50. PMID 3771743.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Markell EK, Udkow MP (1990). "Association of Blastocystis hominis with human disease?". J. Clin. Microbiol. 28 (5): 1085–6. PMID 2351728.
- ^ Zierdt CH (1991). "Pathogenicity of Blastocystis hominis". J. Clin. Microbiol. 29 (3): 662–3. PMID 2037690.
- ^ Rosenblatt JE (1990). "Blastocystis hominis". J. Clin. Microbiol. 28 (10): 2379–80. PMID 2101593.
- ^ a b Lee MJ (1991). "Pathogenicity of Blastocystis hominis". J. Clin. Microbiol. 29 (9): 2089. PMID 1774343.
- ^ a b Carrascosa M, Martínez J, Pérez-Castrillón JL. "Hemorrhagic proctosigmoiditis and Blastocystis hominis infectioqar=1996". PMID 8534017.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ Gupta R, Parsi K (2006). "Chronic urticaria due to Blastocystis hominis". Australas. J. Dermatol. 47 (2): 117–9. doi:10.1111/j.1440-0960.2006.00244.x. PMID 16637808.
- ^ a b c d Amin O. "The epidemiology of Blastocystis hominis in the United States". Research Journal of Parasitology. 1 (1): 1–10.
{{cite journal}}: Cite has empty unknown parameter:|1=(help) - ^ Biedermann T, Hartmann K, Sing A, Przybilla B (2002). "Hypersensitivity to non-steroidal anti-inflammatory drugs and chronic urticaria cured by treatment of Blastocystis hominis infection". Br. J. Dermatol. 146 (6): 1113–4. PMID 12072100.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pasqui AL, Savini E, Saletti M, Guzzo C, Puccetti L, Auteri A (2004). "Chronic urticaria and blastocystis hominis infection: a case report". European review for medical and pharmacological sciences. 8 (3): 117–20. PMID 15368795.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Valsecchi R, Leghissa P, Greco V (2004). "Cutaneous lesions in Blastocystis hominis infection". Acta Derm. Venereol. 84 (4): 322–3. PMID 15339085.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Krüger K, Kamilli I, Schattenkirchner M (1994). "[Blastocystis hominis as a rare arthritogenic pathogen. A case report]". Zeitschrift für Rheumatologie (in German). 53 (2): 83–5. PMID 8023590.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Lee MG, Rawlins SC, Didier M, DeCeulaer K (1990). "Infective arthritis due to Blastocystis hominis". Ann. Rheum. Dis. 49 (3): 192–3. PMID 2322029.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Wilson KW, Winget D, Wilks S (1990). "Blastocystis hominis infection: signs and symptoms in patients at Wilford Hall Medical Center". Military medicine. 155 (9): 394–6. PMID 2120622.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Doyle PW, Helgason MM, Mathias RG, Proctor EM (1990). "Epidemiology and pathogenicity of Blastocystis hominis". J. Clin. Microbiol. 28 (1): 116–21. PMID 2298869.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zaki M, Daoud AS, Pugh RN, al-Ali F, al-Mutairi G, al-Saleh Q (1991). "Clinical report of Blastocystis hominis infection in children". The Journal of tropical medicine and hygiene. 94 (2): 118–22. PMID 2023289.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ O'Gorman MA, Orenstein SR, Proujansky R, Wadowsky RM, Putnam PE, Kocoshis SA (1993). "Prevalence and characteristics of Blastocystis hominis infection in children". Clinical pediatrics. 32 (2): 91–6. PMID 8432086.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nimri L, Batchoun R (1994). "Intestinal colonization of symptomatic and asymptomatic schoolchildren with Blastocystis hominis". J. Clin. Microbiol. 32 (11): 2865–6. PMID 7852590.
- ^ a b El-Shazly AM, Abdel-Magied AA, El-Beshbishi SN, El-Nahas HA, Fouad MA, Monib MS (2005). "Blastocystis hominis among symptomatic and asymptomatic individuals in Talkha Center, Dakahlia Governorate, Egypt". Journal of the Egyptian Society of Parasitology. 35 (2): 653–66. PMID 16083074.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ertug S, Karakas S, Okyay P, Ergin F, Oncu S (2007). "The effect of Blastocystis hominis on the growth status of children". Med. Sci. Monit. 13 (1): CR40-3. PMID 17179909.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Moghaddam DD, Ghadirian E, Azami M (2005). "Blastocystis hominis and the evaluation of efficacy of metronidazole and trimethoprim/sulfamethoxazole". Parasitol. Res. 96 (4): 273–5. doi:10.1007/s00436-005-1363-1. PMID 15915364.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rossignol JF, Kabil SM, Said M, Samir H, Younis AM (2005). "Effect of nitazoxanide in persistent diarrhea and enteritis associated with Blastocystis hominis". Clin. Gastroenterol. Hepatol. 3 (10): 987–91. PMID 16234044.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Armentia A, Méndez J, Gómez A; et al. (1993). "Urticaria by Blastocystis hominis. Successful treatment with paromomycin". Allergologia et immunopathologia. 21 (4): 149–51. PMID 8237719.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Grossman I, Weiss LM, Simon D, Tanowitz HB, Wittner M (1992). "Blastocystis hominis in hospital employees". Am. J. Gastroenterol. 87 (6): 729–32. PMID 1590309.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dunn LA, Boreham PF (1991). "The in-vitro activity of drugs against Blastocystis hominis". J. Antimicrob. Chemother. 27 (4): 507–16. PMID 1856129.
- ^ Gonçalves AQ, Viana Jda C, Pires EM, Bóia MN, Coura JR, Silva EF (2007). "The use of the antifungal agent miconazole as an inhibitor of Blastocystis hominis growth in Entamoeba histolytica/E. dispar cultures". Rev. Inst. Med. Trop. Sao Paulo. 49 (3): 201–2. PMID 17625701.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Markell EK, Udkow MP (1986). "Blastocystis hominis: pathogen or fellow traveler?". Am. J. Trop. Med. Hyg. 35 (5): 1023–6. PMID 3766850.
- ^ Haresh K, Suresh K, Khairul Anus A, Saminathan S (1999). "Isolate resistance of Blastocystis hominis to metronidazole". Trop. Med. Int. Health. 4 (4): 274–7. PMID 10357863.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Yakoob J, Jafri W, Jafri N, Islam M, Asim Beg M (2004). "In vitro susceptibility of Blastocystis hominis isolated from patients with irritable bowel syndrome". Br. J. Biomed. Sci. 61 (2): 75–7. PMID 15250669.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "YAKOOB_2004" was defined multiple times with different content (see the help page). - ^ a b c Boorom, K (2006). Commensal and Pathogenic Blastocystis with Case Studies from Oregon's Willamette Valley. p. 112. ISBN 978-1430309048.
{{cite book}}: Unknown parameter|Publisher=ignored (|publisher=suggested) (help) - ^ White AC (2000). "The disappearing arsenal of antiparasitic drugs". N. Engl. J. Med. 343 (17): 1273–4. PMID 11183360.
- ^ van Hal SJ, Stark DJ, Fotedar R, Marriott D, Ellis JT, Harkness JL (2007). "Amoebiasis: current status in Australia". Med. J. Aust. 186 (8): 412–6. PMID 17437396.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zierdt CH, Swan JC, Hosseini J (1983). "In vitro response of Blastocystis hominis to antiprotozoal drugs". J. Protozool. 30 (2): 332–4. PMID 6631776.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mahmoud MS, Saleh WA (2003). "Secretory and humoral antibody responses to Blastocystis hominis in symptomatic and asymptomatic human infections". Journal of the Egyptian Society of Parasitology. 33 (1): 13–30. PMID 12739797.
- ^ a b c Leelayoova S, Taamasri P, Rangsin R, Naaglor T, Thathaisong U, Mungthin M (2002). "In-vitro cultivation: a sensitive method for detecting Blastocystis hominis". Ann. Trop. Med. Parasitol. 96 (8): 803–7. doi:10.1179/000349802125002275. PMID 12625935.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Suresh K, Smith H (2004). "Comparison of methods for detecting Blastocystis hominis". Eur. J. Clin. Microbiol. Infect. Dis. 23 (6): 509–11. doi:10.1007/s10096-004-1123-7. PMID 15168139.
- ^ a b c d e f g h Stensvold R, Brillowska-Dabrowska A, Nielsen HV, Arendrup MC (2006). "Detection of Blastocystis hominis in unpreserved stool specimens by using polymerase chain reaction". J. Parasitol. 92 (5): 1081–7. PMID 17152954.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Parkar U, Traub RJ, Kumar S; et al. (2007). "Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential". Parasitology. 134 (Pt 3): 359–67. doi:10.1017/S0031182006001582. PMID 17052374.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Termmathurapoj S, Leelayoova S, Aimpun P; et al. (2004). "The usefulness of short-term in vitro cultivation for the detection and molecular study of Blastocystis hominis in stool specimens". Parasitol. Res. 93 (6): 445–7. doi:10.1007/s00436-004-1157-x. PMID 15243800.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Vennila GD, Suresh Kumar G, Khairul Anuar A; et al. (1999). "Irregular shedding of Blastocystis hominis". Parasitol. Res. 85 (2): 162–4. PMID 9934969.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Markell EK (1995). "Is there any reason to continue treating Blastocystis infections?". Clin. Infect. Dis. 21 (1): 104–5. PMID 7578717.
- ^ a b Zierdt CH, Nagy B (1993). "Antibody response to Blastocystis hominis infections". Ann. Intern. Med. 118 (12): 985–6. PMID 8489119.
- ^ a b Tan TC, Suresh KG, Thong KL, Smith HV (2006). "PCR fingerprinting of Blastocystis isolated from symptomatic and asymptomatic human hosts". Parasitol. Res. 99 (4): 459–65. doi:10.1007/s00436-006-0177-0. PMID 16628457.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "TAN_2006" was defined multiple times with different content (see the help page). - ^ a b c d e f Kaneda Y, Horiki N, Cheng XJ, Fujita Y, Maruyama M, Tachibana H (2001). "Ribodemes of Blastocystis hominis isolated in Japan". Am. J. Trop. Med. Hyg. 65 (4): 393–6. PMID 11693890.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Tan, K.S.W. (1986). "Blastocystis in humans and animals: new insights using modern methodologies". Vet. Parasitol. 126: 121–144. PMID 15567582. Cite error: The named reference "TAN_2004" was defined multiple times with different content (see the help page).
- ^ a b c d e Stensvold CR, Suresh GK, Tan KS; et al. (2007). "Terminology for Blastocystis subtypes--a consensus". Trends Parasitol. 23 (3): 93–6. doi:10.1016/j.pt.2007.01.004. PMID 17241816.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Sohail MR, Fischer PR (2005). "Blastocystis hominis and travelers". Travel medicine and infectious disease. 3 (1): 33–8. doi:10.1016/j.tmaid.2004.06.001. PMID 17292002.
- ^ a b c Leelayoova S, Rangsin R, Taamasri P, Naaglor T, Thathaisong U, Mungthin M (2004). "Evidence of waterborne transmission of Blastocystis hominis". Am. J. Trop. Med. Hyg. 70 (6): 658–62. PMID 15211009.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hogue, Theresa (January 14, 2007). "Parasite blamed for growing number of stomach disorders". Corvallis Gazette-Times. Retrieved August 8, 2007.
- ^ a b c Boorom KF (2007). "Is this recently characterized gastrointestinal pathogen responsible for rising rates of inflammatory bowel disease (IBD) and IBD associated autism in Europe and the United States in the 1990s?". Med. Hypotheses. 69 (3): 652–9. doi:10.1016/j.mehy.2007.01.027. PMID 17382484.
- ^ Taamasri P, Mungthin M, Rangsin R, Tongupprakarn B, Areekul W, Leelayoova S (2000). "Transmission of intestinal blastocystosis related to the quality of drinking water". Southeast Asian J. Trop. Med. Public Health. 31 (1): 112–7. PMID 11023076.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lamka KG, LeChevallier MW, Seidler RJ. (1980). Bacterial contamination of drinking water supplies in a modern rural neighborhood. Vol. 39. pp. 734–8. PMID 7377773.
{{cite book}}:|journal=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Li LH, Zhou XN, Du ZW; et al. (2007). "Molecular epidemiology of human Blastocystis in a village in Yunnan province, China". doi:10.1016/j.parint.2007.06.001. PMID 17627869.
{{cite journal}}: Cite journal requires|journal=(help); Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) Cite error: The named reference "LI_2007" was defined multiple times with different content (see the help page). - ^ Utzinger J, Wu Z, Chen JX, Chen SH, Zhang L. (2005). "Viable blastocystis cysts in Scottish and Malaysian sewage samples". Appl Environ Microbiol. 71 (9): 5619–20. PMID 16151162.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zaki M, Zaman V, Sheikh NA (1996). "Resistance of blastocystis hominis cysts to chlorine". JPMA. The Journal of the Pakistan Medical Association. 46 (8): 178–9. PMID 8936976.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Khalifa AM, El Temsahy MM, Abou El Naga IF (2001). "Effect of ozone on the viability of some protozoa in drinking water". Journal of the Egyptian Society of Parasitology. 31 (2): 603–16. PMID 11478459.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Al-Binali AM, Bello CS, El-Shewy K, Abdulla SE. (2006). "The prevalence of parasites in commonly used leafy vegetables in South Western, Saudi Arabia". Saudi Med J. 27 (5): 613–6. PMID 16680247.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Koutsavlis AT, Valiquette L, Allard R, Soto J (2001). "Blastocystis hominis: a new pathogen in day-care centres?". Can. Commun. Dis. Rep. 27 (9): 76–84. PMID 11381629.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Skeels MR, Sokolow R, Hubbard CV, Andrus JK, Baisch J. (1990). Am J Public Health. 80 (3): 305–8. PMID 2305910 http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=2305910.
{{cite journal}}: Missing or empty|title=(help); Text "Cryptosporidium infection in Oregon public health clinic patients 1985-88: the value of statewide laboratory surveillance." ignored (help)CS1 maint: multiple names: authors list (link) - ^ Leder K, Hellard ME, Sinclair MI, Fairley CK, Wolfe R (2005). "No correlation between clinical symptoms and Blastocystis hominis in immunocompetent individuals". J. Gastroenterol. Hepatol. 20 (9): 1390–4. doi:10.1111/j.1440-1746.2005.03868.x. PMID 16105126.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nimri LF (1993). "Evidence of an epidemic of Blastocystis hominis infections in preschool children in northern Jordan". J. Clin. Microbiol. 31 (10): 2706–8. PMID 8253970.
- ^ a b c d Kappus KK, Juranek DD, Roberts JM (1991). "Results of testing for intestinal parasites by state diagnostic laboratories, United States, 1987". MMWR. CDC surveillance summaries : Morbidity and mortality weekly report. CDC surveillance summaries / Centers for Disease Control. 40 (4): 25–45. PMID 1779956.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Basualdo J, Pezzani B, De Luca M, Córdoba A, Apezteguía M (2000). "Screening of the municipal water system of La Plata, Argentina, for human intestinal parasites". Int J Hyg Environ Health. 203 (2): 177–82. PMID 11109572.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Noël C, Dufernez F, Gerbod D; et al. (2005). "Molecular phylogenies of Blastocystis isolates from different hosts: implications for genetic diversity, identification of species, and zoonosis". J. Clin. Microbiol. 43 (1): 348–55. doi:10.1128/JCM.43.1.348-355.2005. PMID 15634993.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b c Tan TC, Suresh KG (2006). "Predominance of amoeboid forms of Blastocystis hominis in isolates from symptomatic patients". Parasitol. Res. 98 (3): 189–93. doi:10.1007/s00436-005-0033-7. PMID 16323025.
- ^ Amin OM (2002). "Seasonal prevalence of intestinal parasites in the United States during 2000". Am. J. Trop. Med. Hyg. 66 (6): 799–803. PMID 12224595.
- ^ Lagacé-Wiens PR, VanCaeseele PG, Koschik C (2006). "Dientamoeba fragilis: an emerging role in intestinal disease". CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 175 (5): 468–9. doi:10.1503/cmaj.060265. PMID 16940260.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Menounos PG, Spanakos G, Tegos N, Vassalos CM, Papadopoulou C, Vakalis NC (2007). "Direct detection of Blastocystis sp. in human faecal samples and subtype assignment using single strand conformational polymorphism and sequencing". doi:10.1016/j.mcp.2007.06.007. PMID 17669623.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ Kukoschke KG, Müller HE (1992). "Varying incidence of Blastocystis hominis in cultures from faeces of patients with diarrhoea and from healthy persons". Zentralbl. Bakteriol. 277 (1): 112–8. PMID 1520961.
- ^ MacPherson DW, MacQueen WM (1994). "Morphological diversity of Blastocystis hominis in sodium acetate-acetic acid-formalin-preserved stool samples stained with iron hematoxylin". J. Clin. Microbiol. 32 (1): 267–8. PMID 7510311.
- ^ Lanuza MD, Carbajal JA, Villar J, Mir A, Borras R. (1999). Parasitol Res. 85 (2): 93–7. PMID 9934956.
{{cite journal}}: Missing or empty|title=(help); Text "Soluble-protein and antigenic heterogeneity in axenic Blastocystis hominis isolates: pathogenic implications." ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Puthia MK, Sio SW, Lu J, Tan KS (2006). "Blastocystis ratti induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells". Infect. Immun. 74 (7): 4114–23. doi:10.1128/IAI.00328-06. PMID 16790785.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ al-Tawil YS, Gilger MA, Gopalakrishna GS, Langston C, Bommer KE (1994). "Invasive Blastocystis hominis infection in a child". Archives of pediatrics & adolescent medicine. 148 (8): 882–5. PMID 8044274.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Long HY, Handschack A, König W, Ambrosch A (2001). "Blastocystis hominis modulates immune responses and cytokine release in colonic epithelial cells". Parasitol. Res. 87 (12): 1029–30. PMID 11763434.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Puthia MK, Vaithilingam A, Lu J, Tan KS (2005). "Degradation of human secretory immunoglobulin A by Blastocystis". Parasitol. Res. 97 (5): 386–9. doi:10.1007/s00436-005-1461-0. PMID 16151742.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ McGowan K, Kane A, Asarkof N; et al. (1983). "Entamoeba histolytica causes intestinal secretion: role of serotonin". Science. 221 (4612): 762–4. PMID 6308760.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ McGowan K, Guerina V, Wicks J, Donowitz M (1985). "Secretory hormones of Entamoeba histolytica". Ciba Found. Symp. 112: 139–54. PMID 2861068.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Banu, Naheed; et al. (2005). "Neurohumoral alterations and their role in amoebiasis" (PDF). Indian J. Clin Biochem. 20 (2): 142–5.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Hussain R, Jaferi W, Zuberi S; et al. (1997). "Significantly increased IgG2 subclass antibody levels to Blastocystis hominis in patients with irritable bowel syndrome". Am. J. Trop. Med. Hyg. 56 (3): 301–6. PMID 9129532.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Giacometti A, Cirioni O, Fiorentini A, Fortuna M, Scalise G (1999). "Irritable bowel syndrome in patients with Blastocystis hominis infection". Eur. J. Clin. Microbiol. Infect. Dis. 18 (6): 436–9. PMID 10442423.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Markell EK, Udkow MP (1986). "Blastocystis hominis: pathogen or fellow traveler?". Am. J. Trop. Med. Hyg. 35 (5): 1023–6. PMID 3766850.
- ^ Brumpt E (1912). "Blastocystis Hominis N. sp et formes voisines". Bull. Soc. Pathol. Exot. 5: 725–730.
- ^ Zaman V, Howe J, Ng M (1995). "Ultrastructure of Blastocystis hominis cysts". Parasitol. Res. 81 (6): 465–9. PMID 7567903.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Moe KT, Singh M, Howe J; et al. (1996). "Observations on the ultrastructure and viability of the cystic stage of Blastocystis hominis from human feces". Parasitol. Res. 82 (5): 439–44. PMID 8738284.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Tan SW, Singh M, Yap EH; et al. (1996). "Colony formation of Blastocystis hominis in soft agar". Parasitol. Res. 82 (4): 375–7. PMID 8740557.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Tan SW, Singh M, Thong KT; et al. (1996). "Clonal growth of Blastocystis hominis in soft agar with sodium thioglycollate". Parasitol. Res. 82 (8): 737–9. PMID 8897510.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link)
External links
[edit]Category:Parasitic diseases Category:Water-borne diseases
Category:Chromista
es:Blastocystis hominis
it:Blastocystis hominis
GOOD LINK FOR TREATMENT NOTES:
http://www.nzetc.org/tm/scholarly/tei-WH2Surg-pt2-c1-2.html
