User:Eg-T2g
Appearance
| Wikipedia:Babel | ||||||
|---|---|---|---|---|---|---|
| ||||||
| Search user languages |
Eg and T2g are 2- en 3-dimensional representations in the 48-fold cubic point group Oh. The g in the subscript denotes inversion symmetry.
Eg and T2g are the representations of the d-orbitals of transition metals with an FCC-crystal structure like copper, gold, platinum and silver.
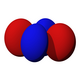
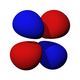
| Eg | T2g | |||
|---|---|---|---|---|
| dz2 | dx2-y2 | dxz | dyz | dxy |
 |
 |
 |
 |

|
The material in the
![]() sandbox and the pipeline
sandbox and the pipeline ![]()
is under construction.
Activities on wiki.riteme.site
[edit]Eg-T2g made contributions to:
- Density of states adding substantial info and reconstruction
- Free electron model adding substantial info
- Empty Lattice Approximation construction
- Nearly-free electron model adding introduction and info
- Tight binding substantial reconstruction
- Electronic band structure some additional remarks
- Self energy some additional remarks
- Friedel oscillations added substantial info
- Spin-orbital
- Slater-type orbital added differentials section
- Atomic orbital minor remarks
- Relativistic quantum chemistry added length contraction remark
- Cubic harmonics construction
- Impurity added thermodynamical remark
- Pigment added info in physical basis introduction and minor remarks
- Conjugated system adding text + info
- Absorption band cleaning, corrections and updating
- Metallic bond added links and info on dielectric function
Books on several subjects
[edit]
|
|
Some interesting science pages
[edit]|
Chemistry and Physics |
Mathematics Biology
|
See also
[edit]
|
references
[edit]- ^ Herbert Goldstein (1980). Classical Mechanics. Addison Wesley. ISBN 0-201-02918-9.
- ^
R. P. Feynman, R. B. Leighton, M. Sands (1963). The Feynman Lectures on Physics. Addison-Wesley Publishing Company. ISBN 0-201-02116-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Albert Messiah (1999). Quantum Mechanics. Dover Publications. ISBN 0-486-40924-4.
- ^ Stephen Gasiorowicz (1974). Quantum Physics. Wiley & Sons. ISBN 0-471-29281-8.
- ^ Eugen Merzbacher (1961). Quantum Mechanics. Wiley & Sons. ISBN 0471596701.
- ^
E. U. Condon & G. H. Shortley (1935–1991). The Theory of Atomic Spectra. Cambridge University Press. ISBN 0-521-09209-4.
{{cite book}}: CS1 maint: date format (link) - ^
Gerhard Herzberg (1944–1987). Atomic Spectra and Atomic Structure. Dover Publications. ISBN 0-471-60115-3.
{{cite book}}: Check|isbn=value: checksum (help)CS1 maint: date format (link) - ^
C. Kittel (1953–1976). Introduction to Solid State Physics. Wiley & Sons. ISBN 0-471-49024-5.
{{cite book}}: CS1 maint: date format (link) - ^ J. M. Ziman (1964). Principles of the Theory of Solids. Cambridge University Press. ISBN 0-521-29733-8.
- ^ W. Jones, N. H. March (1973). Theoretical Solid State Physics. Wiley and Sons - Dover Publications. ISBN 0-486-65015-4.
- ^
W. A. Harrison (1979). Solid State Theory. Dover Publications. ISBN 0-486-638948-7.
{{cite book}}: Check|isbn=value: length (help) - ^ R. Mirman (1999). Point Groups, Space Groups, Crystals, Molecules. World Scientific. ISBN 981-02-3732-4.
- ^ G. Burns (1977). Introduction to Group Theory with Applications. Academic Press. ISBN 0-12-145750-8.
- ^ Walter Ashley Harrison (1989). Electronic Structure and the Properties of Solids. Dover Publications. ISBN 0-486-66021-4.
- ^ R. McWeeny (1978). Methods of Molecular Quantum Mechanics. Academic Press. ISBN 0-12-486552-6.
- ^
Szabo, A & Ostlund, N. S. (1989). Modern Quantum Chemistry. McGraw-Hill. ISBN 0-486-69186-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ P. W. Atkins (1970). Molecular Quantum Mechanics. Oxford University Press. ISBN 0-19-855129-0.
- ^ D. M. Brink & G. R. Satchler (1993). Angular Momentum. Oxford University Press. ISBN 0-19-851759-9.
- ^
A.A. Abrikosov, L.P. Gorkov, I.E. Dzyaloshinsky (1963–1975). Methods of Quantum Field Theory in Statistical Physics. Dover Publications. ISBN 0-486-63228-8.
{{cite book}}: CS1 maint: date format (link) CS1 maint: multiple names: authors list (link) - ^
Richard D. Mattuck (1967–1992). A Guide to Feynman Diagrams in the Many-Body Problem. Dover Publications. ISBN 0-486-67047-3.
{{cite book}}: CS1 maint: date format (link) - ^ E. N. Economou (1983). Green's Functions in Quantum Physics. Springer-Verlag. ISBN 0-387-12266-4.
- ^ Gerald D. Mahan (1990). Many-Particle Physics. Plenum Press. ISBN 0-306-43423-7.
- ^ P. Fulde (1991). Electron Correlations in Molecules and Solids. Springer-Verlag. ISBN 0-387-53623-X.
- ^
C. E. Housecraft, A. G. Sharpe (2005). Inorganic Chemistry. Pearson Education Ltd. ISBN 0-19-039913-2.
{{cite book}}: Check|isbn=value: checksum (help) - ^ M. Clugston, R. Flemming (2000). Advanced Chemistry. Oxford University Press. ISBN 0-19-914633-0.
- ^ Michio Kaku (1993). Quantum Field Theory. Oxford University Press. ISBN 0-19-509158-2.
