User:Doodlepuppy123/Cyclopentadienyl anion
| This is the sandbox page where you will draft your initial Wikipedia contribution.
If you're starting a new article, you can develop it here until it's ready to go live. If you're working on improvements to an existing article, copy only one section at a time of the article to this sandbox to work on, and be sure to use an edit summary linking to the article you copied from. Do not copy over the entire article. You can find additional instructions here. Remember to save your work regularly using the "Publish page" button. (It just means 'save'; it will still be in the sandbox.) You can add bold formatting to your additions to differentiate them from existing content. |
Article Draft for Cyclopentadienyl anion
[edit]copied from [[Cyclopentadienyl anion]]
In chemistry, the cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of [C
5H
5]−
and abbreviated as Cp−. Deprotonation of cyclopentadiene forms the cyclopentadienyl anion. The cyclopentadienyl anion is a ligand which binds to a metal in organometallic chemistry.
Resonance and aromaticity
[edit]The cyclopentadienyl anion is a planar, cyclic, regular-pentagonal ion; it has 6 π-electrons (4n + 2, where n = 1), which fulfills Hückel's rule of aromaticity.[1][2] Each double bond and lone pair contains 2 π-electrons because the lone pair delocalizes into the ring, shown by moving to different positions in its resonance structures.[3][4] The cyclopentadienyl anion is a conjugated system because there are alternating π and 𝜎 bonds.[5]

All carbons in the planar ring are sp2 hybridized and contain a p orbital.[3][6][7] Two p orbitals overlap to form a π bond.
The structure shown is a composite of five resonance contributors in which each carbon atom carries part of the negative charge.[7]
Cyclopentadiene has a pKa of about 16 which is acidic since the conjugate base, cyclopentadienyl anion, has resonance stabilization of the lone pair and negative formal charge.[8]
Ligand Characteristics
[edit]The cyclopentadienyl anion is a polydentate ligand because it has multiple attachment points to a metal.[9][10] The carbon with the lone pair and negative formal charge is an X-type ligand (anionic electron donor) and both double bonds are L-type (neutral electron donors). Since the cyclopentadienyl anion has two L-type ligands and one X-type ligand, it is an L2X ligand.[9][10]
The cyclopentadienyl anion has 5 atoms in an uninterrupted π system that bind to a metal, so it has a maximum hapticity of 5.
Cyclopentadienyl groups are not reactive with nucleophiles or electrophiles, making them good spectator ligands.[11]
Bonding to form complexes
[edit]The cyclopentadienyl anion can bond as a ligand to metal atoms, forming coordination compounds known as cyclopentadienyl complexes. Examples of cyclopentadienyl complexes are ferrocenium, which is an oxidant, and cobaltocene, which is a soluble reductant.[12]
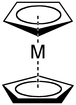

Cyclopentadienyl anions bonded to metals form shorter bonds than neutral aromatic hydrocarbons because they are more electron-rich and therefore better electron donors.[13]
When two cyclopentadienyl anions bind to a metal, they form bis(cyclopentadienyl) complexes.
Bis(cyclopentadienyl) complexes with an oxidation state of two are called metallocenes.[14] An X-type ligand causes the oxidation state to increase by one, so two L2X cyclopentadienyl ligands would increase the oxidation state by two. If there are no other X-type ligands and no overall charge then the oxidation state of the metal would be two. The cyclopentadienyl anions can bind to the metal in different ways. For example, cyclopentadienyl anions bind linearly in ferrocene, either eclipsed or staggered, so the complex will have D5h or D5d symmetry, respectively.[15][16] If the cyclopentadienyl anions bind in a bent shape (less than 180 degrees apart), the symmetry will be C2v or Cs for eclipsed or staggered, respectively.[15]

Cyclopentadienyl zinc complexes show that the cyclopentadienyl anion can bind to the zinc metal center in different ways such that it can have hapticities of 1 to 5.[17] The complex is symmetric when the cyclopentadienyl anion has a hapticity of 5 and is asymmetric when there are intermediate hapticities of 2 or 3.[17]
To synthesize cyclopentadienyl complexes, salts of the cyclopentadienyl anion are reagents. Lithium and sodium cations are commonly used, but potassium cations can also form cyclopentadienide salts.[18] Sodium cyclopentadienide and potassium cyclopentadienide are stable because of the aromatic anion.[19]
Cyclopentadienyl, [C
5H
5]−
, and cyclopentadiene, C
5H
6, can substitute one or more hydrogens, forming derivatives having covalent bonds. (See Cyclopentadiene#Derivatives)
Applications
[edit]Cyclopentadienyl complexes are a potential anticancer therapy. Organometallic C,N-bound Ir(III) cyclopentadienyl complexes exhibit cytotoxicity.[20] The cytotoxicity was increased by substituting biphenyl and phenyl groups to the Cp ligand.[20][21]
Cyclopentadienyl anion influenced Copernicium's symbol
[edit]It was proposed that element 112, copernicium, have the symbol Cp, but Cp- was already the abbreviation for the cyclopentadienyl anion, so copernicium’s elemental symbol is Cn.[22]
References
[edit]- ^ Vikram R. Jadhav; Jamdhade Madhuri; Wadhawane Pooja; Y.R. Baste (2020-11-07). "Cyclopentadienyl System: Solving the Secular Determinant, π Energy, Delocalization Energy, Wave Functions, Electron Density and Charge Density". Journal of Chemistry, Environmental Sciences and its Applications. 6 (2). doi:10.15415/jce.2020.62002. ISSN 2349-7769.
- ^ Rachuru, Sanjeev; Padmavathi, Devarakonda A.; Jagannadham, V. (2023-01-18). "Why Cyclohexatriene (C6H6, pKa = 43) is Less Acidic than Cyclopentadiene (C5H6, pKa = 15) and Cycloheptatriene (C7H8, pKa = 36): A Freshmen Chemical Education Undergraduate Exercise". World Journal of Chemical Education. 11 (1): 1–3. doi:10.12691/wjce-11-1-1. ISSN 2375-1665.
- ^ a b Das, Arijit (2021-06-19). "Classification of Negative Charge Discriminate Hybridization with Aromatic and Anti-aromatic Behavior of Organic Compounds - Innovative Mnemonics". World Journal of Chemical Education. 9 (2): 57–63. doi:10.12691/wjce-9-2-4. ISSN 2375-1665.
- ^ Paul, Satadal; Goswami, Tamal; Misra, Anirban (2015-10-01). "Noncomparative scaling of aromaticity through electron itinerancy". AIP Advances. 5 (10): 107211. doi:10.1063/1.4933191.
- ^ "Delocalised electrons- Definition and Examples of Delocalized electrons with FAQs". BYJUS. Retrieved 2023-04-01.
- ^ Cyclopentadienyl anion, retrieved 2023-04-01
- ^ a b "15.4: Aromatic Ions". Chemistry LibreTexts. 2015-05-03. Retrieved 2023-04-14.
- ^ "Aromatic stability IV (video)". Khan Academy. Retrieved 2023-04-01.
- ^ a b "13.3.2: Simplifying the Organometallic Complex by Deconstruction". Chemistry LibreTexts. 2022-06-14. Retrieved 2023-04-01.
- ^ a b "Simplifying the Organometallic Complex (Part 3)". Chemistry LibreTexts. 2013-10-02. Retrieved 2023-04-14.
- ^ "9.3: Metal Cyclopentadienyl Complexes". Chemistry LibreTexts. 2019-08-15. Retrieved 2023-04-01.
- ^ "Cyclopentadienyl Anion - Introduction, Cyclopentadienyl Anion Complexes, Synthesis and Applications of Cyclopentadienyl Anion". BYJUS. Retrieved 2023-04-01.
- ^ "Cyclopentadienyl Group - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2023-04-02.
- ^ Caliskan, Betul (2017-07-05). Radical Mechanisms in the Metallocenes. IntechOpen. ISBN 978-953-51-3318-6.
- ^ a b Lauher, Joseph W.; Hoffmann, Roald (March 1, 1976). "Structure and chemistry of bis(cyclopentadienyl)-MLn complexes". Journal of the American Chemical Society. 98 (7): 1729–1742. doi:10.1021/ja00423a017. ISSN 0002-7863.
- ^ Mohammadi, Narges; Ganesan, Aravindhan; Chantler, Christopher T.; Wang, Feng (April 10, 2012). "Differentiation of ferrocene D5d and D5h conformers using IR spectroscopy". Journal of Organometallic Chemistry. 713: 51–59. doi:10.1016/j.jorganchem.2012.04.009.
- ^ a b Mebs, Stefan; Chilleck, Maren A.; Grabowsky, Simon; Braun, Thomas (2012-09-10). "Hapticity Uncovered: Real-Space Bonding Indicators for Zincocene Chemistry". Chemistry - A European Journal. 18 (37): 11647–11661. doi:10.1002/chem.201200870.
- ^ Komarov, Pavel D.; Birin, Kirill P.; Vinogradov, Alexander A.; Varaksina, Evgenia A.; Puntus, Lada N.; Lyssenko, Konstantin A.; Churakov, Andrei V.; Nifant’ev, Ilya E.; Minyaev, Mikhail E.; Roitershtein, Dmitrii M. (2022-11-09). "Coordination Polymers of Polyphenyl-Substituted Potassium Cyclopentadienides". Molecules. 27 (22): 7725. doi:10.3390/molecules27227725. ISSN 1420-3049. PMC 9696914. PMID 36431825.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Tkachuk, R.; Lee, C. C. (1959-10-01). "A STUDY ON THE STRUCTURE OF THE CYCLOPENTADIENYL ANION WITH C 14 AS TRACER". Canadian Journal of Chemistry. 37 (10): 1644–1654. doi:10.1139/v59-239. ISSN 0008-4042.
- ^ a b Liu, Zhe; Habtemariam, Abraha; Pizarro, Ana M.; Clarkson, Guy J.; Sadler, Peter J. (2011-09-12). "Organometallic Iridium(III) Cyclopentadienyl Anticancer Complexes Containing C,N-Chelating Ligands". Organometallics. 30 (17): 4702–4710. doi:10.1021/om2005468. ISSN 0276-7333.
- ^ Kar, Binoy; Roy, Nilmadhab; Pete, Sudhindra; Moharana, Prithvi; Paira, Priyankar (2020-11-01). "Ruthenium and iridium based mononuclear and multinuclear complexes: A Breakthrough of Next-Generation anticancer metallopharmaceuticals". Inorganica Chimica Acta. 512: 119858. doi:10.1016/j.ica.2020.119858. ISSN 0020-1693.
- ^ "Copernicium Video - The Periodic Table of Videos - University of Nottingham". www.periodicvideos.com. Retrieved 2023-04-02.
