User:Dmochizuki/sandbox
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Prezista |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607042 |
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 37% (without ritonavir), 82% (with ritonavir) |
| Protein binding | 95% |
| Metabolism | hepatic (CYP3A4) |
| Elimination half-life | 15 hours |
| Excretion | Faeces (80%), urine (14%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| Chemical and physical data | |
| Formula | C27H37N3O7S |
| Molar mass | 547.665 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Darunavir (brand name Prezista, formerly known as TMC114) is a second-generation protease inhibitor used for the treatment of treatment-naïve and treatment-experienced HIV infections.[1] Darunavir can be given as a tablet or liquid.[2]
Darunavir is to be administered with low-dose Ritonavir or Cobicistat, which decrease the metabolism of Darunavir, thus increasing the concentration of the drug.[3] Common side effects include diarrhea, nausea, vomiting, abdominal pain, headache, and rash. [4]
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[5] Developed by pharmaceutical company Tibotec, darunavir is named after Arun K. Ghosh, Ph.D., who discovered the molecule at the University of Illinois at Chicago.[6] It was approved by the Food and Drug Administration (FDA) on June 23, 2006.[7][8]
Medical uses
[edit]Darunavir is an OARAC (DHHS) recommended treatment option for treatment-naïve and treatment-experienced adults and adolescents. It showed comparable efficacy to lopinavir/ritonavir at 96 weeks with a once-daily dosing in treatment-naïve patients.[9] It was approved by the FDA for people not previously treated on October 21, 2008.[10]
Side effects
[edit]As with other antiretrovirals, darunavir does not cure HIV infection or AIDS.
In studies, darunavir was generally well tolerated. Mild to moderate rash was seen in 7% of patients. Some patients developed severe rash. In clinical studies, 0.3% of patients discontinued due to rash. The most common moderate to severe side effects associated with darunavir include diarrhea (2.3%), headache (3.8%), abdominal pain (2.3%), constipation (2.3%), and vomiting (1.5%). Four percent of patients discontinued treatment due to adverse events. People who are allergic to darunavir or any of its ingredients, or ritonavir (Norvir) should not take darunavir.
Relevant drug-drug interactions with other medications commonly used in HIV patient populations were few, such as other antiretroviral medications, proton pump inhibitors, and H2 receptor antagonists. St. John's wort may reduce its effectiveness by interaction with CYP3A. Patients should talk to their healthcare providers about all the medicines they are taking or plan to take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
Before taking darunavir, patients should tell their healthcare providers if they have any medical conditions, including diabetes, liver problems, hemophilia, or allergy to sulfa medicines, and should tell their doctors if they are pregnant or planning to become pregnant, or are nursing. Darunavir should be used with caution in patients with hepatic impairment.
High blood sugar, diabetes or worsening of diabetes, muscle pain, tenderness or weakness, and increased bleeding in people with hemophilia have been reported in patients taking protease inhibitor medicines like darunavir. Changes in body fat have been seen in some patients taking anti-HIV medicines, including loss of fat from legs, arms and face, increased fat in the abdomen and other internal organs, breast enlargement, and fatty lumps on the back of the neck. The cause and long-term health effects of these conditions are not known at this time.
Clinical laboratory safety observed in the darunavir group was comparable to the control group.[11]
Mechanism of action
[edit]Darunavir is a nonpeptidic inhibitor of PR that lodges itself in the active site of PR through a number of hydrogen bonds.[12] It was developed to increase interactions with HIV-1 protease and to be more resistant against HIV-1 protease mutations. With a Kd value of 4.5 x 10−12 M, darunavir has a much stronger interaction with PR and its dissociation constant is 1/100 to 1/1000 of other protease inhibitors.[13] This strong interaction comes from increased hydrogen bonds between darunavir and the backbone of the PR active site (Figure 2). Darunavir’s structure allows it to create more hydrogen bonds with the PR active site than most PIs that have been developed and approved by the FDA.[14] Furthermore, the backbone of HIV-1 protease maintains its spatial conformation in the presence of mutations.[15] Because darunavir interacts with this stable portion of the protease, the PR-PI interaction is less likely to be disrupted by a mutation.[14]
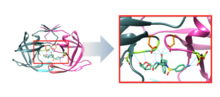
Catalytic site
[edit]The chemical activity of the HIV-1 protease depends on two residues in the active site, Asp25 and Asp25’, one from each copy of the homodimer.[16] Darunavir interacts with these catalytic aspartates and the backbone of the active site through hydrogen bonds, specifically binding to residues Asp25, Asp25’, Asp 29, Asp 30, Asp 30’, and Gly 27 (Figure 3). This interaction prevents viral replication, as it competitively inhibits the viral polypeptides from gaining access to the active site and strongly binds to the enzymatic portions of this protein.[12]
Pharmacoeconomic considerations
[edit]In the US and UK, healthcare costs were estimated to be lower with boosted darunavir than with investigator-selected control protease inhibitors in treatment-experienced patients.[17]
History
[edit]
The development of first-generation clinical inhibitors was founded on creating more protease-ligand interactions through hydrogen bonding and hydrophobic interactions.[12] The first HIV protease inhibitor approved by the FDA was saquinavir, which was designed to target wild-type HIV-1 protease.[18] However, this inhibitor is no longer effective due to resistance-causing mutations on the HIV-1 protease structure. The HIV genome has high plasticity, so has been able to become resistant to multiple HIV-1 protease inhibitors.[19] Since saquinavir, the FDA has approved several PIs, including darunavir.[20] Darunavir was granted approval by the FDA on June 23, 2006, and is one of the most recently developed protease inhibitors approved for treatment of HIV.[20]
See also
[edit]References
[edit]- ^ Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents, November 3, 2008, Developed by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC). full guidelines.
- ^ "Darunavir: MedlinePlus Drug Information". medlineplus.gov. Retrieved 2016-11-10.
- ^ "Darunavir Monograph for Professionals - Drugs.com". www.drugs.com. Retrieved 2016-11-10.
- ^ Madruga, José Valdez; Berger, Daniel; McMurchie, Marilyn; Suter, Fredy; Banhegyi, Denes; Ruxrungtham, Kiat; Norris, Dorece; Lefebvre, Eric; de Béthune, Marie-Pierre (2007-07-13). "Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial". The Lancet. 370 (9581): 49–58. doi:10.1016/S0140-6736(07)61049-6. PMID 17617272. S2CID 26084893.
- ^ "19th WHO Model List of Essential Medicines (April 2015)" (PDF). WHO. April 2015. Retrieved May 10, 2015.
- ^ http://www.chem.purdue.edu/ghosh/
- ^ Rodger D MacArthura, Darunavir: promising initial results, doi:10.1016/S0140-6736(07)60499-1
- ^ "2006 - FDA Approves New HIV Treatment for Patients Who Do Not Respond to Existing Drugs". www.fda.gov. Retrieved 2016-11-10.
- ^ hivandhepatitis.com, Efficacy and Safety of Boosted Darunavir (Prezista) Are Superior to Lopinavir/ritonavir (Kaletra) at 96 Weeks: ARTEMIS Trial, 2008-10-28, URL.
- ^ hivandhepatitis.com, Darunavir (Prezista) Receives Full Traditional Approval, Dose Set for Treatment-naive Patients, Caution Urged for Pregnant Women, 2008-10-24, URL.
- ^ (Product Monograph, Darunavir)
- ^ a b c Leonis, G., Czyznikowska, Z. et al. "Computational Studies of Darunavir into HIV-1 Protease and DMPC Bilayer: Necessary Conditions for Effective Binding and the Role of the Flaps" J. Chem. Inf. Model. 2012, 52, 1542-1558.
- ^ King, N. M., Prabu-Jeyabalan, M. et al. "Structural and Thermodynamic Basis for the Binding of TMC114, a Next-Generation Human Immunodeficiency Virus Type 1 Protease Inhibitor" Journal of Virology. 2004, vol. 78 no. 21
- ^ a b Lefebvre, E., Schiffer, C. A. "Resilience to Resistance of HIV-1 Protease Inhibitors: Profile of Darunavir" AIDS Rev. 2008; 10(3): 131-142
- ^ Lascar, R. M., Benn, P. "Role of darunavir in the management of HIV infection" HIV AIDS (Auckl). 2009; 1:31-39.
- ^ Li, D., Zhang, Y. et al. "Investigation on the mechanism for the binding and drug resistance of wild type and mutations of G86 residue in HIV-1 protease complexed with Darunavir by molecular dynamic simulation and free energy calculation" J. Molecular Modeling, 2014, 20:2122.
- ^ Liu, F., Kovalevsky, A.Y. "Effect of Flap Mutations on Structure of HIV-1 Protease and Inhibition by Saquinavir and Darunavir" J. Mol. Biol. 2008; 381(1): 102-115.
- ^ Eron, J. "HIV-1 Protease Inhibitors" Oxford Journal of Clinical Infectious Diseases. 2000, vol. 30, Issue Supplement 2, 160-170.
- ^ a b "HIV/AIDS Historical Time Line 2000-2010" FDA. 2011.
External links
[edit]- Darunavir FAQ at AIDSmeds.com
- Drug information in PDF
- Arun Ghosh group
- Tibotec
[[Category:Protease inhibitors]] [[Category:Sulfonamides]] [[Category:Carbamates]] [[Category:Belgian inventions]] [[Category:World Health Organization essential medicines]]
