User:Davidjpod/5mC sandbox
Sandbox page for 5mC - current page is in italics. Modifications will be added in non italics.
5-Methylcytosine is a methylated form of the DNA base cytosine that may be involved in the regulation of gene transcription. When cytosine is methylated, the DNA maintains the same sequence, but the expression of methylated genes can be altered (the study of this is known as epigenetics). 5-Methylcytosine is incorporated in the nucleoside 5-methylcytidine.
In 5-methylcytosine, a methyl group is attached to the 5th atom in the 6-atom ring (counting counterclockwise from the NH nitrogen at the six o'clock position, not the 2 o'clock). This methyl group distinguishes 5-methylcytosine from cytosine.
Discovery
[edit]While trying to isolate the bacterial toxin responsible for tuberculosis, W.G. Ruppel isolated a novel nucleic acid named tuberculinic acid in 1898 from Tubercle bacillus.[1] The nucleic acid was found to be unusual in that it contained, in addition to thymine, guanine and cytosine, a methylated nucleotide. In 1925, Johnson and Coghill successfully detected a minor amount of a methylated cytosine derivative as a product of hydrolysis of tuberculinic acid with sulfuric acid.[2][3] This report was severely criticized because their identification based solely on the optical properties of the crystalline picrate, and other scientists failed to reproduce the same result.[4] But its existence was ultimately proven in 1948, when Hotchkiss separated the nucleic acids of DNA from calf thymus using paper chromatography. With this method he detected a unique methylated cytosine, quite distinct from conventional cytosine and uracil.[5] After seven decades, it turned out that it is also a common feature in different RNA molecules, although its precise role is uncertain.[6]
In vivo
[edit]5-Methylcytosine is an epigenetic modification formed by the action of DNA methyltransferases.

The function of this chemical varies significantly among species:[7]
- In bacteria, 5-methylcytosine can be found at a variety of sites, and is often used as a marker to protect DNA from being cut by native methylation-sensitive restriction enzymes.
- In plants, 5-methylcytosine occurs at CpG, CpHpG and CpHpH sequences (where H = A, C or T).
- In fungi and animals, 5-methylcytosine predominantly occurs at CpG dinucleotides. Most eukaryotes methylate only a small percentage of these sites, but 70-80% of CpG cytosines are methylated in vertebrates. In mammalian cells, clusters of CpG at the 5' ends of genes are termed CpG islands [8]. 1% of all mammalian DNA is 5mC[9].
While spontaneous deamination of cytosine forms uracil, which is recognized and removed by DNA repair enzymes, deamination of 5-methylcytosine forms thymine. This conversion of a DNA base from cytosine (C) to thymine (T) can result in a transition mutation.[10] In addition, active enzymatic deamination of cytosine, or 5-methylcytosine by the APOBEC family of cytosine deaminases, could have beneficial implications on various cellular processes as well as on organismal evolution.[11] The implications of deamination on 5-hydroxymethylcytosine, on the other hand, remains less understood.
In vitro
[edit]The NH2 group can be removed (deamination) from 5-methylcytosine to form thymine with use of reagents such as nitrous acid; cytosine deaminates to uracil under similar conditions.
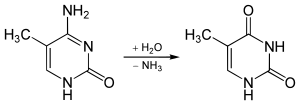
5-methylcytosine is resistant to deamination by bisulfite treatment, which deaminates cytosine residues. This property is often exploited to analyze DNA cytosine methylation patterns with bisulfite sequencing.[12]
Addition and Regulation with DNMTs
[edit]5mC marks are placed on genomic DNA via DNA methyltransferases (DNMTs). There are 5 DNMTs in humans: DNMT1, DNMT2, DNMT3A, DNMT3B, and DNMT3L; in algae and fungi 3 more are present: DNMT4, DNMT5, and DNMT6. [13] DNMT1 contains the replication foci targeting sequence (RFTS) and the CXXC domains which catalyze the addition of 5mC marks. RFTS targets (directs instead of "targets"?) DNMT1 to loci of DNA replication to assist in the maintenance of 5mC on the strands during DNA replication, whereas CXXC contains a zinc finger domain for de novo addition of methylation to the DNA[14]. DNMT1 was found to be the predominant DNA methyltransferase in all human tissue[15]. DNMT3A and DNMT3B are responsible for de novo methylation whereas DNMT1 is for maintaining 5mC through replication[16]. DNMTs have been found to interact with each other to increase methylating capability. For example, 2 DNMT3L can bind with 2 DNMT3A in a complex to improve interactions with DNA to facilitate the methylation. [17] Changes in the expression of DNMT1, the de novo methyltransferase, results in aberrant methylation. Over expression produces increased methylation, whereas disruption of the enzyme decreased levels of methylation[15]. (Does this mean that methyltransferase expression is tightly regulated?) (Perhaps a bit more context on how this experiment was performed would also add to the clarity of this information.)
The mechanism of the addition is (as follows:)(Despite the admonitions of my fourth grade teacher, I think it is stylistically ok to have a colon follow "is".) first a cysteine residue at the DNMT's PCQ motif creates a nucleophillic attack at carbon 6 on the cysteine nucleotide that is to be methylated. S-Adenosylmethionine donates a methyl group to carbon 5. A base in the enzyme deprotonates the residual hydrogen on carbon 5 restoring the double bond between carbon 5 and 6 in the ring, producing the 5-methylcytosine base pair.[14] (Perhaps a figure would be helpful to illustrate this chemistry.)(<- Agreed)
Demethylation
[edit]After a cytosine is methylated to 5mC, it can be reversed back to its initial state via multiple mechanisms. Passive DNA demethylation by dilution acts to reverses diminishes the mark by it being lost gradually through replication by a lack of maintenance by DNMT1. (This sentence is a bit unclear to me) 5mC is actively reversed (termed In active DNA demethylation,) by a series of oxidations converting converts it to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC), the latter of which are eventually excised by thymine DNA glycosylase (TDG), followed by base excision repair (BER) to restore the cytosine[16]. TDG knockout produced a 2-fold increase of 5fC without any statistically significant change to levels of 5hmC, indicating 5mC must be iteratively oxidized at least twice before its active demethylation[18]. The oxidation occurs through the TET (Ten-eleven translocation) family dioxygenases that can convert 5mC, 5hmC, and 5fC to their oxidized forms. However the enzyme has the greatest preference for 5mC and the initial reaction rate for 5hmC and 5fC conversions with TET2 has have been seen to be 4.9-7.6 fold slower[19]. TET requires Fe(II) as cofactor, and oxygen and α-ketoglutarate (α-KG) as substrates, the latter of which is generated from isocitrate by the enzyme isocitrate dehydrogenase (IDH)[20]. Cancer however can produce 2-hydroxyglutarate (2HG) which competes with α-KG, thus reducing TET activity, in turn reducing conversion of 5mC to 5hmC[21]. (Nice transition to next section.)
Role of 5mC in Cancer
[edit]In cancer, DNA becomes both overly methylated (hypermethlyation) termed hypermethylation, and under-methylated (hypomethylation) termed hypomethylation[22]. (Might be nice to specify which cancers you're talking about. Or is this found in all cancers?) CpG islands overlapping gene promoters can be hypermethylated are de novo methylated in cancer <_-I'd keep "in cancer" resulting in aberrant inactivation of genes normally associated with growth inhibition, resulting in tumor formation an example of hypermethylation[23]. Comparing tumor and normal tissue, the former had elevated levels of the methyltransferases DNMT1, DNMT3A, and mostly DNMT3B, all of which are associated with the abnormal levels of 5mC in cancer. [15] Repeat sequences in the genome including satellite DNA, Alu, and long interspersed elements (LINE) are often seen hypomethylated in cancer, resulting in expression of these normally repressed genes, and levels are often significant markers of tumor progression. [22] It has been hypothesized that there a connection between the hypermethylation and hypomethylation; over activity of DNA methyltransferases that produce the abnormal de novo 5mC methylation may be compensated by the removal of methylation, a type of epigenetic repair. However the removal of methylation is inefficient resulting in an overshoot of genome-wide hypomethylation. The contrary may also be possible that over expression of hypomethylated satellites may be silenced by genome-wide hypermethylation. [22] Cancer hallmark capabilities are likely acquired through epigenetic changes that alter the 5mC in the both cancer cells and in surrounding tumor-associated stroma within the tumor microenvironment.[24]
Role of 5mC as a Biomarker of Aging
[edit]There is a connection between chronological age and levels of DNA methylation in the genome, producing the concept of "epigenetic age".[25] (This first sentence is a fragment. are you missing a word?) Coupling levels of DNA methylation in specific sets of CpGs, called "clock CpGs", with algorithms that regress typical levels of collective genome-wide methylation at a given chronological age, allows for age predication based on epigenetic changes age prediction of genetic material in cells, tissues, etc. During youth (0-20 years old), changes in DNA methylation occur at a faster rate as development and growth progresses, and the changes begin to slow down at older ages. Multiple epigenetic age estimators exist. Horvath's clock measures a blood & multi-tissue set of 353 CpGs, half of which positively correlate with age, and the other half negatively, to estimate the epigenetic age.[26] Hannum's clock utilizes adult blood samples to calculate age based on an orthogonal basis of 71 CpGs.[27] Levine's clock, known as DNAm PhenoAge, depends on 513 CpGs and surpasses the other age estimators in predicting mortality and lifespan, yet displays bias with non blood tissues.[28] There are reports of age estimators with the methylation state of only one CpG in the gene ELOVL2[29]. Estimation of age allows for prediction lifespan by being able to expect the emergence of which age related conditions that individual may be subject to based off their 5mC methylation markers.
Peer Review/Feedback
[edit]This reads well overall. I added a few comments/revisions relating to wording/phrasing. Another suggestion is to make the last two sections subsections under a larger section titled "Roles of 5mC in humans" or "Biological role of 5mC". Should "de novo" be italicized?
I think this reads really well — I made a few small edits throughout. I especially like the sections on cancer and aging. In the cancer section, I think it might be nice to add a line or two talking about how methylation affects different types of cancers etc. In the aging section, which I think is great, it might nice to include a couple line talking about some of the new data on epigenetics and aging. For example, there are new data showing that treating cells with certain Yamanaka factors reverses some age-related epigenetic changes. There is also a growing body of evidence that suggests epigenetic changes don't just correlate with aging, but actually drive some of the aging process. Here's some more on this topic:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4736728/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6351826/
https://www.nature.com/articles/s41556-018-0206-0
References:
[edit]- ^ Matthews AP (2012). Physiological Chemistry. Williams & Wilkins Company/. p. 167. ISBN 1130145379.
- ^ Johnson TB, Coghill RD (1925). "The discovery of 5-methyl-cytosine in tuberculinic acid, the nucleic acid of the Tubercle bacillus". J Am Chem Soc. 47 (11): 2838–2844. doi:10.1021/ja01688a030.
- ^ Grosjean H (2009). Nucleic Acids Are Not Boring Long Polymers of Only Four Types of Nucleotides: A Guided Tour. Landes Bioscience.
- ^ Vischer E, Zamenhof S, Chargaff E (1949). "Microbial nucleic acids: the desoxypentose nucleic acids of avian tubercle bacilli and yeast". J Biol Chem. 177 (1): 429–438. PMID 18107446.
- ^ Hotchkiss RD (1948). "The quantitative separation of purines, pyrimidines and nucleosides by paper chromatography". J Biol Chem. 175 (1): 315–332. PMID 18873306.
- ^ Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T (2012). "Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA". Nucleic Acids Res. 40 (11): 5023–5033. doi:10.1093/nar/gks144. PMC 3367185. PMID 22344696.
- ^ Colot V, Rossignol JL (1999). "Eukaryotic DNA methylation as an evolutionary device". BioEssays. 21 (5): 402–411. doi:10.1002/(SICI)1521-1878(199905)21:5<402::AID-BIES7>3.0.CO;2-B. PMID 10376011.
- ^ Bird, Adrian P. (1986-05). "CpG-rich islands and the function of DNA methylation". Nature. 321 (6067): 209–213. doi:10.1038/321209a0. ISSN 1476-4687.
{{cite journal}}: Check date values in:|date=(help) - ^ Ehrlich, M.; Wang, R. Y. (1981-06-19). "5-Methylcytosine in eukaryotic DNA". Science. 212 (4501): 1350–1357. doi:10.1126/science.6262918. ISSN 0036-8075. PMID 6262918.
- ^ Sassa A, Kanemaru Y, Kamoshita N, Honma M, Yasui M (2016). "Mutagenic consequences of cytosine alterations site-specifically embedded in the human genome". Genes and Environ. 38 (1): 17. doi:10.1186/s41021-016-0045-9. PMC 5007816. PMID 27588157.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Chahwan R, Wontakal SN, Roa S (2010). "Crosstalk between genetic and epigenetic information through cytosine deamination". Trends in Genetics. 26 (10): 443–448. doi:10.1016/j.tig.2010.07.005. PMID 20800313.
- ^ Clark SJ, Harrison J, Paul CL, Frommer M (1994). "High sensitivity mapping of methylated cytosines". Nucleic Acids Res. 22 (15): 2990–2997. doi:10.1093/nar/22.15.2990. PMC 310266. PMID 8065911.
- ^ Ponger, Loïc; Li, Wen-Hsiung (2005-04-01). "Evolutionary Diversification of DNA Methyltransferases in Eukaryotic Genomes". Molecular Biology and Evolution. 22 (4): 1119–1128. doi:10.1093/molbev/msi098. ISSN 0737-4038.
- ^ a b Lyko, Frank (2018-02). "The DNA methyltransferase family: a versatile toolkit for epigenetic regulation". Nature Reviews Genetics. 19 (2): 81–92. doi:10.1038/nrg.2017.80. ISSN 1471-0064.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Robertson, K D; Uzvolgyi, E; Liang, G; Talmadge, C; Sumegi, J; Gonzales, F A; Jones, P A (1999-06-01). "The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors". Nucleic Acids Research. 27 (11): 2291–2298. ISSN 0305-1048. PMID 10325416.
- ^ a b Wu, Xiaoji; Zhang, Yi (2017-05-30). "TET-mediated active DNA demethylation: mechanism, function and beyond". Nature Reviews Genetics. 18 (9): 517–534. doi:10.1038/nrg.2017.33. ISSN 1471-0056.
- ^ Jia, Da; Jurkowska, Renata Z.; Zhang, Xing; Jeltsch, Albert; Cheng, Xiaodong (2007-09). "Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation". Nature. 449 (7159): 248–251. doi:10.1038/nature06146. ISSN 1476-4687.
{{cite journal}}: Check date values in:|date=(help) - ^ Song, Chun-Xiao; Szulwach, Keith E.; Dai, Qing; Fu, Ye; Mao, Shi-Qing; Lin, Li; Street, Craig; Li, Yujing; Poidevin, Mickael; Wu, Hao; Gao, Juan (2013-04-25). "Genome-wide profiling of 5-formylcytosine reveals its roles in epigenetic priming". Cell. 153 (3): 678–691. doi:10.1016/j.cell.2013.04.001. ISSN 1097-4172. PMC 3657391. PMID 23602153.
- ^ Ito, Shinsuke; Shen, Li; Dai, Qing; Wu, Susan C.; Collins, Leonard B.; Swenberg, James A.; He, Chuan; Zhang, Yi (2011-09-02). "Tet Proteins Can Convert 5-Methylcytosine to 5-Formylcytosine and 5-Carboxylcytosine". Science. 333 (6047): 1300–1303. doi:10.1126/science.1210597. ISSN 0036-8075. PMID 21778364.
- ^ Lu, Xingyu; Zhao, Boxuan Simen; He, Chuan (2015-02-12). "TET Family Proteins: Oxidation Activity, Interacting Molecules, and Functions in Diseases". Chemical Reviews. 115 (6): 2225–2239. doi:10.1021/cr500470n. ISSN 0009-2665.
- ^ Xu, Wei; Yang, Hui; Liu, Ying; Yang, Ying; Wang, Ping; Kim, Se-Hee; Ito, Shinsuke; Yang, Chen; Wang, Pu; Xiao, Meng-Tao; Liu, Li-xia (2011-01-18). "Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of α-Ketoglutarate-Dependent Dioxygenases". Cancer cell. 19 (1): 17–30. doi:10.1016/j.ccr.2010.12.014. ISSN 1535-6108. PMC 3229304. PMID 21251613.
- ^ a b c Ehrlich, Melanie (2009-12-01). "DNA hypomethylation in cancer cells". Epigenomics. 1 (2): 239–259. doi:10.2217/epi.09.33. ISSN 1750-1911. PMC 2873040. PMID 20495664.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Jones, Peter A. (1996-06-01). "DNA Methylation Errors and Cancer". Cancer Research. 56 (11): 2463–2467. ISSN 0008-5472. PMID 8653676.
- ^ Hanahan, Douglas; Weinberg, Robert A. (2011-03-04). "Hallmarks of Cancer: The Next Generation". Cell. 144 (5): 646–674. doi:10.1016/j.cell.2011.02.013. ISSN 0092-8674. PMID 21376230.
- ^ Horvath, Steve; Raj, Kenneth (2018-06). "DNA methylation-based biomarkers and the epigenetic clock theory of ageing". Nature Reviews Genetics. 19 (6): 371–384. doi:10.1038/s41576-018-0004-3. ISSN 1471-0064.
{{cite journal}}: Check date values in:|date=(help) - ^ Horvath, Steve (2013-12-10). "DNA methylation age of human tissues and cell types". Genome Biology. 14 (10): 3156. doi:10.1186/gb-2013-14-10-r115. ISSN 1474-760X. PMC 4015143. PMID 24138928.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Hannum, Gregory; Guinney, Justin; Zhao, Ling; Zhang, Li; Hughes, Guy; Sadda, SriniVas; Klotzle, Brandy; Bibikova, Marina; Fan, Jian-Bing; Gao, Yuan; Deconde, Rob (2013-01-24). "Genome-wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates". Molecular cell. 49 (2): 359–367. doi:10.1016/j.molcel.2012.10.016. ISSN 1097-2765. PMC 3780611. PMID 23177740.
- ^ Levine, Morgan E.; Lu, Ake T.; Quach, Austin; Chen, Brian H.; Assimes, Themistocles L.; Bandinelli, Stefania; Hou, Lifang; Baccarelli, Andrea A.; Stewart, James D.; Li, Yun; Whitsel, Eric A. (2018-04-17). "An epigenetic biomarker of aging for lifespan and healthspan". Aging (Albany NY). 10 (4): 573–591. doi:10.18632/aging.101414. ISSN 1945-4589. PMC 5940111. PMID 29676998.
- ^ Garagnani, Paolo; Bacalini, Maria G.; Pirazzini, Chiara; Gori, Davide; Giuliani, Cristina; Mari, Daniela; Blasio, Anna M. Di; Gentilini, Davide; Vitale, Giovanni; Collino, Sebastiano; Rezzi, Serge (2012). "Methylation of ELOVL2 gene as a new epigenetic marker of age". Aging Cell. 11 (6): 1132–1134. doi:10.1111/acel.12005. ISSN 1474-9726.
