User:Cuine100/sandbox
| Ferrochelatase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Human ferrochelatase | |||||||||
| Identifiers | |||||||||
| EC no. | 4.99.1.1 | ||||||||
| CAS no. | 9012-93-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Ferrochelatase is an enzyme that is encoded by the FECH gene in humans.[1] Ferrochelatase catalyses the terminal (eighth) step in the biosynthesis of heme, converting protoporphyrin IX into heme B. It catalyses the reaction:
- protoporphyrin + Fe+2 ↔ heme B + 2 H+
Function
[edit]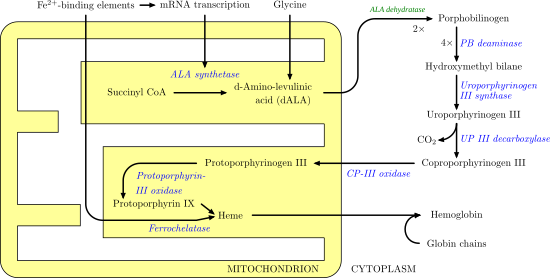
Ferrochelatase catalyzes the insertion of ferrous iron into protoporphyrin IX in the heme biosynthesis pathway to form heme B. The enzyme is localized to the matrix-facing side of the inner mitochondrial membrane. Ferrochelatase is the best known member of a family of enzymes that add divalent metal cations to tetrapyrrole structures.[2] For example, magnesium chelatase adds magnesium to protoporphyrin IX in the first step of bacteriochlorophyll biosynthesis.
Heme B is an essential cofactor in many proteins and enzymes. In particular, heme b plays a key role as the oxygen carrier in hemoglobin in red blood cells and myoglobin in muscle cells. Furthermore, heme B is found in cytochrome b, a key component in Q-cytochrome c oxidoreductase (complex III) in oxidative phosphorylation.[3]
Structure
[edit]| Ferrochelatase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Ferrochelatase | ||||||||
| Pfam | PF00762 | ||||||||
| InterPro | IPR001015 | ||||||||
| PROSITE | PDOC00462 | ||||||||
| SCOP2 | 1ak1 / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 137 | ||||||||
| OPM protein | 1hrk | ||||||||
| |||||||||
Human ferrochelatase is a homodimer composed of two 359 amino acid polypeptide chains. It has a total molecular weight of 85.07 kDa.[4] Each subunit is composed of five regions: a mitochondrial localization sequence, the N terminal domain, two folded domains, and a C terminal extension. Residues 1-62 form a mitochondrial localization domain that is cleaved in post-translational modification. The folded domains contain a total of 17 α-helices and 8 β-sheets. The C terminal extension contains three of the four cysteine residues (Cys403, Cys406, Cys411) that coordinate the catalytic iron-sulfur cluster (2Fe-2S). The fourth coordinating cysteine resides in the N-terminal domain (Cys196).[5]
The active pocket of ferrocheltase consists of two hydrophobic "lips" and a hydrophilic interior. The hydrophobic lips face the inner mitochondrial membrane and facilitate the passage of the poorly soluble protoporphyrin IX substrate and the heme product via the membrane.[5]
Mechanism
[edit]The exact mechanism of human protoporphyrin metallation is not perfectly understood; nevertheless, data from other organisms provide some insight. Researchers studying Bacillus subtilis ferrochelatase propose a mechanism for iron insertion into protoporphyrin in which the enzyme tightly grips rings B, C, and D while bending ring A 36o. Normally planar, this distortion exposes the lone pair of electrons on the nitrogen in ring A to the Fe+2 ion.[2] A highly conserved histidine residue (His183) is essential for the distortion, as well as metallation and possibly proton extraction. Subsequent investigation revealed a 100o distortion in protoporphyrin bound to human ferrochelatase.[6]
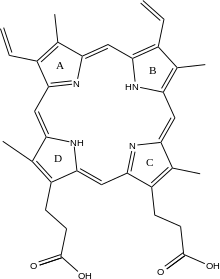
Clinical significance
[edit]Defects in ferrochelatase create a buildup of protoporphyrin IX, causing erythropoietic protoporphyria (EPP).[7] The disease can result from a variety of mutations in FECH, most of which behave in an autosomal dominant manner with low clinical penetrance. Clinically, patients with EPP present with a range of symptoms, from asymptomatic to suffering from an extremely painful photosensitivity. In less than five percent of cases, accumulation of protoporphyrin in the liver results in cholestasis (blockage of bile flow from the liver to the small intestine) and terminal liver failure.[8]
Interactions
[edit]Ferrochelatase has been shown to interact with ABCB7.[9]
See also
[edit]References
[edit]- ^ http://www.uniprot.org/uniprot/P22830
- ^ a b Lecerof, D.; Fodje, M.; Hansson, A.; Hansson, M.; Al-Karadaghi, S. (March 2000). "Structural and mechanistic basis of porphyrin metallation by ferrochelatase". Journal of Molecular Biology. 297 (1): 221–232. doi:doi:10.1006/jmbi.2000.3569.
{{cite journal}}: Check|doi=value (help) - ^ Berg, Jeremy; Tymoczko, John; Stryer, Lubert (2012). Biochemistry (7th ed. ed.). New York: W.H. Freeman. ISBN 9781429229364.
{{cite book}}:|edition=has extra text (help) - ^ http://www.rcsb.org/pdb/explore/explore.do?structureId=1HRK
- ^ a b Wu, Chia-Kuei; Dailey, Harry A.; Rose, John P.; Burden, Amy; Sellers, Vera M.; Wang, Bi-Cheng (1 February 2001). "The 2.0 Å structure of human ferrochelatase, the terminal enzyme of heme biosynthesis". Nature Structural Biology. 8 (2): 156–160. doi:10.1038/84152.
{{cite journal}}:|access-date=requires|url=(help) - ^ Karlberg, Tobias; Hansson, Mattias D.; Yengo, Raymond K.; Johansson, Renzo; Thorvaldsen, Hege O.; Ferreira, Gloria C.; Hansson, Mats; Al-Karadaghi, Salam (May 2008). "Porphyrin Binding and Distortion and Substrate Specificity in the Ferrochelatase Reaction: The Role of Active Site Residues". Journal of Molecular Biology. 378 (5): 1074–1083. doi:doi:10.1016/j.jmb.2008.03.040.
{{cite journal}}: Check|doi=value (help) - ^ James, William D.; Berger, Timothy G. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 0-7216-2921-0.
- ^ Rüfenacht, U.B.; Gouya, L.; Schneider-Yin, X.; Puy, H.; Schäfer, B.W.; Aquaron, R.; Nordmann, Y.; Minder, E.I.; Deybach, J.C. (1998). "Systematic Analysis of Molecular Defects in the Ferrochelatase Gene from Patients with Erythropoietic Protoporphyria". The American Journal of Human Genetics. 62 (6): 1341–52. doi:10.1086/301870. PMC 1377149. PMID 9585598.
- ^ Taketani S, Kakimoto K, Ueta H, Masaki R, Furukawa T (Apr 2003). "Involvement of ABC7 in the biosynthesis of heme in erythroid cells: interaction of ABC7 with ferrochelatase". Blood. 101 (8): 3274–80. doi:10.1182/blood-2002-04-1212. PMID 12480705.
Further reading
[edit]- Cox TM (Jun 1997). "Erythropoietic protoporphyria". Journal of Inherited Metabolic Disease. 20 (2): 258–69. doi:10.1023/A:1005317124985. PMID 9211198.
- Buller RE, Schrader WT, O'Maller BW (May 1976). "Steroids and the practical aspects of performing binding studies". Journal of Steroid Biochemistry. 7 (5): 321–6. doi:10.1016/0022-4731(76)90090-X. PMID 180343.
- Bonkowsky HL, Bloomer JR, Ebert PS, Mahoney MJ (Nov 1975). "Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts". The Journal of Clinical Investigation. 56 (5): 1139–48. doi:10.1172/JCI108189. PMC 301976. PMID 1184741.
- Brenner DA, Didier JM, Frasier F, Christensen SR, Evans GA, Dailey HA (Jun 1992). "A molecular defect in human protoporphyria". American Journal of Human Genetics. 50 (6): 1203–10. PMC 1682545. PMID 1376018.
- Nakahashi Y, Fujita H, Taketani S, Ishida N, Kappas A, Sassa S (Jan 1992). "The molecular defect of ferrochelatase in a patient with erythropoietic protoporphyria". Proceedings of the National Academy of Sciences of the United States of America. 89 (1): 281–5. doi:10.1073/pnas.89.1.281. PMC 48220. PMID 1729699.
- Lamoril J, Boulechfar S, de Verneuil H, Grandchamp B, Nordmann Y, Deybach JC (Dec 1991). "Human erythropoietic protoporphyria: two point mutations in the ferrochelatase gene". Biochemical and Biophysical Research Communications. 181 (2): 594–9. doi:10.1016/0006-291X(91)91231-Z. PMID 1755842.
- Diep A, Li C, Klisak I, Mohandas T, Sparkes RS, Gaynor R, Lusis AJ (Dec 1991). "Assignment of the gene for cyclic AMP-response element binding protein 2 (CREB2) to human chromosome 2q24.1-q32". Genomics. 11 (4): 1161–3. doi:10.1016/0888-7543(91)90047-I. PMID 1838349.
- Nakahashi Y, Taketani S, Okuda M, Inoue K, Tokunaga R (Dec 1990). "Molecular cloning and sequence analysis of cDNA encoding human ferrochelatase". Biochemical and Biophysical Research Communications. 173 (2): 748–55. doi:10.1016/S0006-291X(05)80099-3. PMID 2260980.
- Rossi E, Attwood PV, Garcia-Webb P, Costin KA (May 1990). "Inhibition of human lymphocyte ferrochelatase activity by hemin". Biochimica Et Biophysica Acta. 1038 (3): 375–81. doi:10.1016/0167-4838(90)90251-A. PMID 2340297.
- Polson RJ, Lim CK, Rolles K, Calne RY, Williams R (Sep 1988). "The effect of liver transplantation in a 13-year-old boy with erythropoietic protoporphyria". Transplantation. 46 (3): 386–9. doi:10.1097/00007890-198809000-00010. PMID 3047929.
- Bonkovsky HL, Schned AR (Jan 1986). "Fatal liver failure in protoporphyria. Synergism between ethanol excess and the genetic defect". Gastroenterology. 90 (1): 191–201. PMID 3940245.
- Prasad AR, Dailey HA (Aug 1995). "Effect of cellular location on the function of ferrochelatase". The Journal of Biological Chemistry. 270 (31): 18198–200. doi:10.1074/jbc.270.31.18198. PMID 7629135.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Sarkany RP, Alexander GJ, Cox TM (Jun 1994). "Recessive inheritance of erythropoietic protoporphyria with liver failure". Lancet. 343 (8910): 1394–6. doi:10.1016/S0140-6736(94)92525-9. PMID 7910885.
- Tugores A, Magness ST, Brenner DA (Dec 1994). "A single promoter directs both housekeeping and erythroid preferential expression of the human ferrochelatase gene". The Journal of Biological Chemistry. 269 (49): 30789–97. PMID 7983009.
- Maruyama K, Sugano S (Jan 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Dailey HA, Sellers VM, Dailey TA (Jan 1994). "Mammalian ferrochelatase. Expression and characterization of normal and two human protoporphyric ferrochelatases". The Journal of Biological Chemistry. 269 (1): 390–5. PMID 8276824.
- Wang X, Poh-Fitzpatrick M, Carriero D, Ostasiewicz L, Chen T, Taketani S, Piomelli S (Apr 1993). "A novel mutation in erythropoietic protoporphyria: an aberrant ferrochelatase mRNA caused by exon skipping during RNA splicing". Biochimica Et Biophysica Acta. 1181 (2): 198–200. doi:10.1016/0925-4439(93)90112-e. PMID 8481408.
- Nakahashi Y, Miyazaki H, Kadota Y, Naitoh Y, Inoue K, Yamamoto M, Hayashi N, Taketani S (May 1993). "Molecular defect in human erythropoietic protoporphyria with fatal liver failure". Human Genetics. 91 (4): 303–6. doi:10.1007/BF00217346. PMID 8500787.
- Imoto S, Tanizawa Y, Sato Y, Kaku K, Oka Y (Jul 1996). "A novel mutation in the ferrochelatase gene associated with erythropoietic protoporphyria". British Journal of Haematology. 94 (1): 191–7. doi:10.1046/j.1365-2141.1996.d01-1771.x. PMID 8757534.
- Crouse BR, Sellers VM, Finnegan MG, Dailey HA, Johnson MK (Dec 1996). "Site-directed mutagenesis and spectroscopic characterization of human ferrochelatase: identification of residues coordinating the [2Fe-2S] cluster". Biochemistry. 35 (50): 16222–9. doi:10.1021/bi9620114. PMID 8973195.
External links
[edit]- UMich Orientation of Proteins in Membranes protein/pdbid-1hrk
- Ferrochelatase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)





