User:Brianshapiro/Drafts/Creosote
Creosote is the portion of chemical products obtained by the distillation of a tar that remains heavier than water, notably useful for its anti-septic and preservative properties.[1] It is produced in some quantities from the burning of wood and coal in blast furnaces and fireplaces; commonly found inside chimney flues when the wood or coal burns incompletely, producing soot and tarry smoke, and is the compound responsible for the preservation and the flavor of meat in the process of smoking. The name is derived from the Greek kréas (κρέας), meaning "flesh", and sōtēr (σωτήρ), meaning "preserver".[2]
The two main types in industrial production are wood-tar creosote and coal-tar creosote — the coal-tar variety, having stronger and more toxic properties, has chiefly been used as a preservative for wood; while the wood-tar variety has been used for meat preservation, wood treatment, and for medicinal purposes as an expectorant, anti-septic, astringent, anaesthetic and laxative, though these have mostly been replaced by newer medicines. Coal-tar creosote had also in the past been used as an escharotic to burn malignant skin tissue and in dentistry to prevent necrosis, but no longer is used that way because of its toxic, carcinogenic properties and because better and safer treatments are now available. Varieties of creosote have also been made from both petroleum and oil shale called oil-tar creosote, when derived from the oil tar, and water-gas-tar creosote when derived from the water gas tar. Creosote also has been made from pre-coal formations such as lignite, giving to lignite-tar creosote and peat, giving to peat-tar creosote.
Creosotes are commercially valuable, and therefore are produced industrially on a large scale, either for direct use, or as raw material for the production or extraction of various chemicals. There are several other names for such fluids, but most are not trustworthy, being regional, applying to only some variants, or to other fluids as well. For example, pitch oil is used both for some kinds of creosote-like fluids and for kerosene.
Creosote oils
[edit]For some part of their history, wood-tar creosote and coal-tar creosote were suggested to be the same substance — only in different purities — which gives to their common name; the two were only determined to be chemically different substances later on. All types of creosote are composed of phenol derivatives and share some amount of simple phenols,[3] but they aren't the only active element of any of them. For its useful effect, wood-tar creosote relies on the presence of methyl ethers of phenol, and coal-tar creosote on the presence of naphthalenes and anthracenes; otherwise each of them would dissolve in water.
Creosote was first discovered in its wood-tar form in 1832 by Carl Reichenbach, when he found it both in the tar and pyrolingeous acids obtained by a dry distillation of beechwood. Because pyroligneous acid was known as an anti-septic and meat preservative, Reichenbach did experiments with dipping meat in diluted solution of distilled creosote. He found that the meat was dried without undergoing putrefaction and had a delicious, smoky flavor.[4] This led him to reason that creosote was the anti-septic principle contained in smoke, and further argued the creosote he found in wood-tar was also in coal-tar, animal-tar, and amber-tar in the same abundance as in wood-tar.[2]
Soon after, in 1834, Friedrich Ferdinand Runge discovered carbolic acid in coal-tar, and Auguste Laurence obtained it from phenylhydrate, which was soon determined to be the same compound. There was no clear view on the relationship between carbolic acid and creosote; Runge described it as having similar caustic and anti-septic properties, but noted that it was different, in that it was an acid and formed salts. Nonetheless, Reichenbach argued that creosote was also the active element, as it was in pyroligneous acid. Despite evidence to the contrary, his view held sway with most chemists, and it became commonly accepted wisdom that creosote, carbolic acid, and phenylhydrate were identical substances, with different degrees of purity.[2]
Carbolic acid was soon commonly sold under the name "creosote", and the rarity of wood-tar creosote in some places led chemists to believe that it was the same substance as described by Reichenbach. In the 1840s, Eugen Freiherr von Gorup-Besanez after realizing that two samples of substances labeled as creosote were different, started a series of investigations to determine the chemical nature of carbolic acid, leading to a conclusion that it more resembled chlorinated quinones and must have been a different, entirely unrelated substance. Independently, there were investigations into the chemical nature of creosote. A study by F.K. Völkel revealed that purified creosote resembled the smell of guaiacol, and later studies by Heinrich Hlasiwetz determined an element common in guaiacum and cresote which he called creosol, and that creosote contained a mixture of creosol and guaiacol. Later investigations by Gorup-Besanez, A.E. Hoffmann and Siegfried Marasse had shown that wood-tar creosote also contained phenols, giving it a feature in common with coal-tar creosote.[5]
Historically, coal-tar creosote has been distinguished from what was thought of creosote proper — what would refer to the original substance of Reichenbach's discovery — and referred to specifically as "creosote oil". But because creosote from coal-tar and wood-tar are obtained from a similar process and have some common uses, they've also been seen in the same class of substances, with the terms "creosote" or "creosote oil" referring to either product.[1]
Wood-tar creosote
[edit]
| ||||||||||||||||||||||||||||||
Wood-tar creosote is a colourless to yellowish greasy liquid with a smoky odor, produces a sooty flame when burned, and has a burned taste. It is buoyant, with a specific gravity of 1.037 to 1.087, retains fluidity at a very low temperature, and boils at 205-225°C. When transparent, it is in its purest form. Dissolution in water requires up to 200 times the amount of water as the base creosote.[9] The creosote is a combination of plant phenolics: primarily guaiacol and creosol (4-methylguaiacol), which will typically constitute 50% of the oil; second in prevalence, cresol and xylenol; the rest being a combination of monophenols and polyphenols.
|
The simple phenols are not the only valued element in wood-tar creosote; on their own they coagulate albumin, the water-soluble proteins contained in meat, so serve as a preserving agent, but also cause disintegration.[11] Most of the phenols in the creosote are methoxy derivatives — they contain the methoxy group linked to the benzene nucleus (O–CH3). The level of high methyl derivates created from the action of heat on wood (also apparent in the methyl alcohol produced through distillation) make wood-tar creosote substantially different from coal-tar creosote. Guaiacol is a methyl ether of pyrocatechin, while creosol is a methyl ether of methyl-pyrocatechin, the next homolog of pyrocatechin. Methyl ethers differ from simple phenols in that they're less soluble in water, and less caustic and poisonous.[12] This allows meat to successfully be preserved without disintegration, and allows creosote to as a medical treatment.[11]
|
|
Because wood-tar creosote is used for its guaiacol and creosol content, its generally derived from beechwood rather than other woods, since it distills with a higher proportion of those chemicals to other phenolics. The creosote can be obtained by distilling the wood tar, and treating the fraction heavier than water with a sodium hydroxide solution. The alkaline solution would then separated from the insoluble oily layer, boiled upon contact with air to reduce impurities, and decomposed by diluted sulphuric acid. This produces a crude creosote, which would be purified by re-solution in alkali and re-precipitation with acid, and then redistilled with the fraction passing over between 200° and 225° constituting the purified creosote.[14]
When ferric chloride is added to a dilute solution, it will turn green; a characteristic of ortho-oxy derivatives of benzene.[12] It dissolves in sulphuric acid to a red liquid, which slowly changes to purple-violet. Shaken with hydrochloric acid in the absence of air, it becomes red, the color changing in the presence of air to dark brown or black.[11]
In preparation of food by smoking, guaiacol contributes mainly to the smoky taste, while the dimethyl ether of pyrogallol, syringol, is the main chemical responsible for the smoky aroma.
Coal-tar creosote
[edit]
|
Coal-tar creosote is greenish-brown liquid, with different degrees of darkness, viscosity, and fluorescence depending on how its made. When freshly made, the creosote is a yellow oil with a greenish cast and highly fluorescent; the fluorescence increased by exposure to air and light. After settling, the oil is dark green by reflected light and dark red by transmitted light.[17] To the naked eye, it will generally appear brown. The creosote (often called "creosote oil") consists almost wholly of aromatic hydrocarbons, with some amount of bases and acids and other neutral oils. The flash point is 70–75°C and burning point is 90–100°C,[18] and when burned it releases a greenish smoke.[19] The smell largely depends on the naptha content in the creosote; if there is a high amount, it will have a naptha-like smell; otherwise it will smell more of tar.
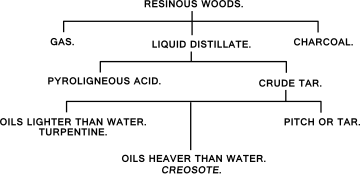
In the process of coal-tar distillation, the distillate is collected into four fractions; the "light oil", which remains lighter than water, the "middle oil" which passes over when the light oil is removed; the "heavy oil", which sinks; and the "anthracene oil", which when cold is mostly solid and greasy, of a buttery consistence. Creosote refers to the portion of coal tar which distills as "heavy oil", typically between 230–270°C, also called "dead oil"; it sinks into water but still is fairly liquid. Carbolic acid is produced in the second fraction of distillation and is often distilled into what is referred to as "carbolic oil".[20][21][22][23]
|
|
Commercial creosote will contain substances from six groups.[15] The two groups occur in the greatest amounts and are the products of the distillation process — the "tar acids", which distill below 205°C and consist mainly of phenols, cresols, and xylenols, including carbolic acid — and aromatic hydrocarbons, which divide into naphthalenes, which distill approximately between 205° and 255°C, and constituents of an anthracene nature, which distill above 255°C.[25] The quantity of each varies based on the quality of tar and temperatures used, but generally, the tar acids won't exceed 5%, the naphthalenes will make up 15 to 50%, and the anthracenes will make up 45% to 70%.[25] The hydrocarbons are mainly aromatic; derivatives of benzene and related cyclic compounds such as naphthalene, anthracene, phenanthrene, acenapthene, and fluorine. Creosotes from vertical-retort and low temperature tars contain, in addition, some paraffinic and olefinic hydrocarbons. The tar-acid content also depends on the source of the tar — it may be less than 3% in creosote from coke-oven tar and as high as 32% in creosote from vertical retort tar.[26] All of these have anti-septic properties. The tar acids are the strongest anti-septics but have the highest degree of solubility in water and are the most volatile; so, like with wood-tar creosote, phenols are not the most valued component, as by themselves they would lend to being poor preservatives.[27] In addition, creosote will contain several products naturally occurring in coal — nitrogen-containing heterocycles, such as acridines, carbazoles, and quinolines, referred to as the "tar bases" and generally make up about 3% of the creosote — sulfur-containing heterocycles, generally benzothiophenes[28] — and oxygen-containing heterocycles, dibenzofurans.[29] Lastly, creosote will contain a small number of aromatic amines produced by the other substances during the distillation process and likely resulting from a combination of thermolysis and hydrogenation.[30][31] The tar bases are often extracted by washing the creosote with aqueous mineral acid,[26] although they're also suggested to have anti-septic ability similar to the tar acids.
Commercially used creosote is often treated to extract the carbolic acid, naphthalene, or anthracene content. The carbolic acid or naphthalene is generally extracted to be used in other commercial products.[32] American produced creosote oils typically will have low amounts of anthracene and high amounts of naphthalene, because when forcing the distillate at a temperature that produces anthracene the soft pitch will be ruined and only the hard pitch will remain; this ruins it for use in roofing purposes, and only leaves a product which isn't commercially useful.[31]
Health effects
[edit]According to the Agency for Toxic Substances and Disease Registry (ATSDR), eating food or drinking water contaminated with high levels of coal tar creosote may cause a burning in the mouth and throat, and stomach pains. ATSDR also states that brief direct contact with large amounts of coal tar creosote may result in a rash or severe irritation of the skin, chemical burns of the surfaces of the eyes, convulsions and mental confusion, kidney or liver problems, unconsciousness, and even death. Longer direct skin contact with low levels of creosote mixtures or their vapors can result in increased light sensitivity, damage to the cornea, and skin damage. Longer exposure to creosote vapors can cause irritation of the respiratory tract.
The International Agency for Research on Cancer (IARC) has determined that coal tar creosote is probably carcinogenic to humans, based on adequate animal evidence and limited human evidence. It is instructive to note that the animal testing relied upon by IARC involved the continuous application of creosote to the shaved skin of rodents. After weeks of creosote application, the animals developed cancerous skin lesions and in one test, lesions of the lung. The United States Environmental Protection Agency has stated that coal tar creosote is a probable human carcinogen based on both human and animal studies.[33] As such, the Federal Occupational Safety and Health Administration (OSHA) has set a permissible exposure limit of 0.2 milligrams of coal tar creosote per cubic meter of air (0.2 mg/m3) in the workplace during an 8-hour day, and the Environmental Protection Agency (EPA) requires that spills or accidental releases into the environment of one pound (0.454 kg) or more of creosote be reported to them.[34]
There is no unique exposure pathway of children to creosote. Children exposed to creosote will probably experience the same health effects seen in adults exposed to creosote. It is unknown whether children differ from adults in their susceptibility to health effects from creosote.
A 2005 mortality study of creosote workers found no evidence supporting an increased risk of cancer death, as a result of exposure to creosote. Based on the findings of the largest mortality study to date of workers employed in creosote wood treating plants, there is no evidence that employment at creosote wood-treating plants or exposure to creosote-based preservatives was associated with any significant mortality increase from either site-specific cancers or non-malignant diseases. The study consisted of 2,179 employees at eleven plants in the United States where wood was treated with creosote preservatives. Some workers began work in the 1940s to 1950s. The observation period of the study covered 1979- 2001. The average length of employment was 12.5 years. One third of the study subjects were employed for over 15 years.[35]
The largest health effect of creosote is deaths caused by residential fires.[36]
Oil-tar creosote
[edit]|
|
Oil-tar creosote is derived from the tar that forms when using petroleum or shale oil in the manufacturing of gas. The distillation of the tar from the oil occurs at very high temperatures; around 980°C. The tar forms at the same time as the gas, and once processed for creosotes contains a high percentage of cyclic hydrocarbons, a very low amount of tar acids and tar bases, and no true anthracenes have been identified.[37] Historically, this has mainly been produced in the United States in the Pacific coast, where petroleum has been more abundant than coal. Limited quantities have been used industrially, either alone, mixed with coal-tar creosote, or fortified with pentachlorophenol.[38]
Water-gas-tar creosote
[edit]Water-gas-tar creosote is also derived from petroleum oil or shale oil, but by a different process; its distilled during the production of water-gas. The tar is a by-product resulting from enrichment of water gas with gases produced by thermal decomposition of petroleum. Of the creosotes derived from oil, its practically the only one used for wood preservation. It has the same degree of solubility as coal-tar creosote and is easy to impregnate into wood. Like standard oil-tar creosote, it has a low amount of tar acids and tar bases, and has less anti-septic qualities.[24] Petri dish tests have shown that water-gas-tar creosote is one-sixth as anti-septically effective as that of that of coal-tar.[39]
Lignite-tar creosote
[edit]Lignite-tar creosote is produced from lignite rather than bituminous coal, and varies considerably from coal-tar creosote. Also called "lignite oil", it has a very high content of tar acids, and has been used to increase the tar acids in normal creosote when necessary.[40] When it has been produced, its generally been applied in mixtures with coal-tar creosote or petroleum. Its effectiveness when used alone has not been established. In an experiment with southern yellow pine fence posts in Mississippi, straight lignite-tar creosote was giving good results after about 27 years exposure, although not as good as the standard coal-tar creosote used in the same situation.[41]
Peat-tar creosote
[edit]There have also been attempts to distill creosote from peat-tar, but they've been mostly unsuccessful, due to the problems with winning and drying peat on an industrial scale.[42] Peat tar by itself has in the past been used as a wood preservative.
Build-up in chimneys
[edit]Burning wood and fossil fuels at low temperature causes incomplete combustion of the oils in the wood, which are off-gassed as volatiles in the smoke. As the smoke rises through the chimney it cools, causing water, carbon, and volatiles to condense on the interior surfaces of the chimney flue. The black oily residue that builds up is referred to as creosote, which is similar in composition to the commercial products by the same name, but with a higher content of carbon black.
Over the course of a season creosote deposits can become several inches thick. This creates a compounding problem, because the creosote deposits reduce the draft (airflow through the chimney) which increases the probability that the wood fire is not getting enough air to burn at high temperature. Since creosote is highly combustible, a thick accumulation creates a fire hazard. If a hot fire is built in the stove or fireplace, and the air control left wide open, this may allow hot oxygen into the chimney where it comes in contact with the creosote which then ignites—causing a chimney fire. Chimney fires often spread to the main building because the chimney gets so hot that it ignites any combustible material in direct contact with it, such as wood. The fire can also spread to the main building from sparks emitting from the chimney and landing on combustible roof surfaces. In order to properly maintain chimneys and heaters that burn wood or carbon-based fuels, the creosote buildup must be removed. Chimney sweeps perform this service for a fee.[36]
73% of heating fires and 25% of all residential fires in the United States are caused by failure to clean out creosote buildup. Since 1990 the number of creosote caused fires has decreased in the United States by 75%.[36] This is partly due to the use of efficient wood-burning stoves that fully burn the volatiles in the smoke, and partly due to the decrease in the use of wood heating during two decades of milder Winter weather, and low fuel prices.[citation needed]
Uses
[edit]Historical
[edit]Industrial
[edit]Soon after it was discovered and recognized as the principle of meat smoking, wood-tar creosote became used as a replacement for the process. Several methods were used to apply the creosote. One was to dip the meat in pyroligneous acid or a water of diluted creosote, as Reichenbach did, or brush it over with them, and within one hour the meat would have the same quality of that of traditionally smoked preparations.[43] Sometimes the creosote was diluted in vinegar rather than water, as vinegar was also used as a preservative.[44] Another was to place the meat in a closed box, and place with it a few drops of creosote in a small bottle. Because of the volatility of the creosote, the atmosphere was filled with a vapor containing it, and it would cover the flesh.[43]
The application of wood tar to sea-going vessels was practiced through the 18th century and early 19th century, before the creosote was isolated as a compound. Wood-tar creosote wasn't found to be as effective in wood treatments, because it was harder to impregnate the creosote into the wood cells, but still experiments[45] were done, including by many governments, because it proved to be less expensive on the market.[46]
The use of coal-tar creosote on a commercial scale began in 1838, when a patent covering the use of creosote oil to treat timber was taken out by John Bethell in 1838. The process he used became referred to as the "Bethell process" and later became more widely known as the full-cell process. It involves sealing wood in a pressure chamber and applying a vacuum to remove air and moisture from the wood cells. The wood is then pressure treated in order to impregnate it with the preservative chemicals. Afterwards the vacuum is applied again to separate the excess solution from the timber. The same year a method to treat wood using zinc choloride was patented by Sir William Burnett and referred to as the "Burnett process".[47] One of the chief uses of creosoted wood was in preserving railway ties (sleepers) so they didn't need to be replaced due to wood rot.
Besides treating wood, it was also used for lighting and fuel. In the beginning, it was only used for lighting needed in harbor and outdoor work, where the smoke that was produced from burning it was of little inconvenience. By 1879, lamps had been created that ensured a more complete combustion by using compressed air, removing the drawback of the smoke. Creosote was also processed into gas and used for lighting that way. As a fuel, it was used to power ships at sea and blast furnaces for different industrial needs, once it was discovered to be more efficient than unrefined coal or wood. It was also used industrially for the softening of hard pitch, and burned produce lamp black. By 1890, the production of creosote in the United Kingdom totaled approximately 29,900,000 gallons per year.[19]
In 1854, Alexander McDougall and Angus Smith developed and patented a product called McDougall's Powder as a sewer deodorant; it was mainly composed from carbolic acid derived from creosote. McDougall in 1864, experimented with his solution to remove entozoa parasites from cattle pasturing on a sewage farm.[48] This later led to widespread use of creosote as a cattle wash and sheep dip. External parasites would be killed in a creosote diluted dip, and drenching tubes would be used to administer doses to the animals stomach to kill internal parasites.[49]
Two later methods for creosoting wood were introduced after the turn of the century, referred to as empty-cell processes, because they involve compressing the air inside the wood so that the preservative can only coat the inner cell walls rather than saturating the interior cell voids. This is a less effective, though usually satisfactory, method of treating the wood, but is used because it requires less of the creosoting material. The first method, the "Rüping process" was patented in 1902, and the second, the "Lowry process" was patented in 1906. Later in 1906, the "Allardyce process" and "Card process" were patented to treat wood with a combination of both creosote and zinc chloride.[47] In 1912, it was estimated that a total of 150,000,000 gallons were produced in the United States per year.
Medical
[edit]Even before creosote as a chemical compound was discovered, it was the chief active component of medicinal remedies in different cultures around the world.
Larrea tridentata, or the so-called creosote bush, as named after its distinct creosote smell, was used by Native Americans in the Southwest as a treatment for many maladies. The Cohahuillia Indians used the plant for intestinal complaints and tuberculosis, the Pima would drink a decoction of the leaves as an emetic, and applied the boiled leaves as poultices to wounds or sores.[50] Popago Indians prepared it medicinally for stiff limbs, snake bites, and menstrual cramps.[51] Guaiacum, after which the guaiacol in creosote was named, was used by native Caribbean islanders to treat tropical diseases and later for syphilis.[52][53]
During antiquity, pitches and resins were used commonly as medicines. Pliny mentions a variety of tar-like substances being used as medicine, including cedria and pissinum.[54] Cedria was the pitch and resin of the cedar tree, being equivalent to the oil of tar and pyrolingeous acid which are used in the first stage of distilling creosote.[55][56] He recommends cedria to ease the pain in a toothache, as an injection in the ear in case of hardness of hearing, to kill parasitic worms, as a preventative for impregnation, as a treatment for phthiriasis and porrigo, as an antidote for the poison of the sea hare, as a liniment for elephantiasis, and as an ointment to treat ulcers both on the skin and in the lungs.[56] He further speaks of cedria being used as the embalming agent for preparing mummies.[54] Pissinum was a tar water that was made by boiling cedria, spreading wool fleeces over the vessels to catch the steam and then wringing it out.[57][58]
The Pharmacopeé of Lyons, published in 1786, says that cedar tree oil can induce vomiting, and suggests it helps medicate tumors and ulcers.[59][60] Physicians contemporary to the discovery of creosote recommended ointments and pills made from tar or pitch to treat skin diseases.[59] Tar water had been used as a folk remedy since the Middle Ages to treat affections like dyspepsia. Bishop Berkeley wrote several works on the medical virtues of tar water, including a philosophical work in 1744 titled Siris: a chain of philosophical reflexions and inquiries concerning the virtues of tar water, and divers other subjects connected together and arising one from another, and a poem where he praised its virtues.[61] Pyroligneous acid was also used at the time in a medicinal water called Aqua Binelli.[59]
Given this history, and the anti-septic properties known to creosote, it became popular among physicians in the 19th century. A dilution of creosote in water was sold in pharmacies as Aqua creosoti, as suggested by the previous use of pyroligneous acid. It was prescribed to quell the irritability of the stomach and bowels and detoxify, treat ulcers and abscesses, neutralize bad odors, and stimulate the mucous tissues of the mouth and throat.[62][63] Creosote in general was listed as an irritant, styptic, anti-septic, narcotic, and diuretic, and in small doses when taken internally as a sedative and anaesthetic. It was used to treat ulcers, and as a way to sterilize the tooth and deaden the pain in case of a tooth-ache.[62]
Creosote was suggested as a treatment for tuberculosis by Reichenbach as soon as 1833. Following Reichenbach, it was argued for by John Elliotson and Sir John Rose Cormack.[62] Elliotson, inspired by the use of creosote to arrest vomiting during an outbreak of cholera, suggested its use for tuberculosis through inhalation. He also suggested it for epilepsy, neuralgia, diabetes and chronic glanders.[64] The idea of using it for tuberculosis failed to take hold, and use of this purpose was dropped, until the idea was revived later in 1876 by the British doctor G. Anderson Imlay, who suggested it be applied locally in spray to the bronchial mucous membrane.[62][65][66] This was followed up in 1877 when it was argued for in a clinical paper by Charles Bouchard and Henri Gimbert.[67] Germ theory had been established by Pasteur in 1860, and Bouchard, arguing that a bacillus was responsible for the disease, sought to rehabilitate creosote for its use as an anti-septic to treat it. He began a series of trials with Gimbert to convince the scientific community, and claimed a promising cure rate.[68] A number of publications in Germany confirmed his results in the following years.[67]
Following that, that was a period of experimentation of different techniques and chemicals using creosote in tuberculosis, which lasted until about 1910, when radiation therapy looked to be a more promising treatment. Guaiacol, instead of a full creosote solution, was suggested by Hermann Sahli in 1887; he argued it had the active chemical of creosote and had the advantage of being of definite composition, and with less of a less unpleasant taste and odor.[69] A number of solutions of both creosote and guaiacol appeared on the market, such as phosphotal and guaicophosphal, phosphites of creosote and guaiacol; eosot and geosot, valerinates of creosote and guaicol; phosot and taphosot, phosphate and tannophospate of creosote; and creosotal and tanosal, tannates of creosote.[70] Creosote and eucalptus oil were also a remedy used together, administered through a vaporizor and inhaler. Since then, more effective and safer treatments for tuberculosis have been developed.
In the 1940s, Canadian-based Eldon Boyd experimented with guaiacol and a recent synthetic modification — glycerol guaiacolate (guaifenesin) — on animals. His data showed that both drugs were effective in increasing secretions into the airways in laboratory animals, when high enough doses were given.
Coal-tar creosote, despite its toxicity, was used as a stimulant and escharotic, as a caustic agent used to treat ulcers and malignancies and cauterize wounds and prevent infection and decay. It was particularly used in dentistry, used to destroy tissues and arrest necrosis[71][72][73]
Current
[edit]Industrial
[edit]Wood-tar creosote is to some extent used for wood preservation, but generally mixed with coal-tar creosote, since it isn't as effective. Commercially available preparations of "liquid smoke", marketed to add a smoked flavor to meat and aid as a preservative, consist primarily of creosote and other constituents of smoke.[74] Creosote is the element which gives liquid smoke its function; guaicol lends to the taste and the creosote oils help act as the preservative.
Coal-tar creosote is the most widely used wood treatment today; both industrially, processed into wood using pressure methods such as "fuel-cell process" or "empty-cell process", and more commonly applied to wood through brushing. In addition to toxicity to fungi, insects, and marine borers, it serves as a natural water repellant. Its commonly used to preserve and waterproof cross ties, pilings, telephone poles, power line poles, marine pilings, and fence posts. Although suitable for use in preserving the structural timbers of buildings, it is not generally used that way because it is difficult to apply.
Due to its carcinogenic character, the European Union has regulated the quality of creosote for the EU market [75] and requires that the sale of creosote be limited to professional users.[76][77] The United States Environmental Protection Agency regulates the use of coal tar creosote as a wood preservative under the provisions of the Federal Insecticide, Fungicide, and Rodenticide Act. Creosote is considered a restricted-use pesticide and is only available to licensed pesticide applicators[78][79]
Medical
[edit]The guaifenesin developed by Eldon Boyd is still commonly used today as an expectorant, sold over the counter, and usually taken by mouth to assist the bringing up of phlegm from the airways in acute respiratory tract infections. Guaifenesin is a component of Mucinex, Robitussin DAC, Cheratussin DAC, Robitussin AC, Cheratussin AC, Benylin, DayQuil Mucous Control, Meltus, and Bidex 400.
Seirogan is a popular Kampo medicine in Japan, used as an anti-diarrheal, and has 133 mg wood creosote from beech, pine, maple or oak wood per adult dose as its primary ingredient. Seirogan was first used as a gastrointestinal medication by the Royal Japanese Army in Russia during the Russo-Japanese War of 1904-5.[80] Creomulsion is a cough medicine in the United States, introduced in 1925, that is still sold and contains beechwood creosote.
Creosote, in the form of samples from the creosote bush, is often found as a herbal remedy and supplement under the name chaparral, and in the form of beechwood creosote under the name kreosotum or kreosote.
Notes
[edit]- ^ a b Price, Kelogg & Cox 1909, p. 7
- ^ a b c Schorlemmer 1885, p. 152
- ^ Roscoe & Schorlemmer 1888, p. 37
- ^ Roscoe & Schorlemmer 1888, p. 33
- ^ Schorlemmer 1885, p. 153
- ^ a b Allen 1910, p. 353
- ^ American Pharmaceutical Association 1894, p. 1073
- ^ Royal Chemical Society 1895, p. 294
- ^ Thorpe 1890, p. 614
- ^ Lee et al. 2005, p. 1483
- ^ a b c Allen 1910, p. 348
- ^ a b Pharmaceutical Society of Great Britain 1898, p. 468
- ^ Price, Kelogg & Cox 1909, p. 13
- ^ Allen 1910, p. 347
- ^ a b Melber, Kielhorn & Mangelsdorf 2004, p. 11
- ^ Speight 1994, p. 456
- ^ Allen 1910, p. 366
- ^ Bateman 1922, p. 50
- ^ a b Thorpe 1890, p. 615
- ^ Philips 1891, p. 255
- ^ Martin 1913, pp. 416–419
- ^ Nelson 1907, p. 204
- ^ Noller 1965, p. 185
- ^ a b c Price, Kelogg & Cox 1909, p. 12
- ^ a b Engineering and Contracting 1912, p. 531
- ^ a b Greenhow 1965, p. 58
- ^ American Railway Bridge and Building Association 1914, p. 287
- ^ Orr & White 2002, p. 39
- ^ Speight 1994, p. 77
- ^ Orr & White 2002, p. 255
- ^ a b Bateman 1922, p. 47
- ^ Mushrush & Speight 1995, p. 115
- ^ EPA 1988
- ^ LOSH 2003
- ^ Wong 2005, pp. 683–697
- ^ a b c DHS 2006
- ^ Voorhies 1940
- ^ Hunt & Garratt 1967, p. 88
- ^ Stimson 1915, p. 626
- ^ Richardson 1993, p. 103
- ^ Hunt & Garratt 1967, p. 97
- ^ Encyclopaedia Britannica 1949, p. 821
- ^ a b Abel & Smith 1857, p. 23
- ^ Letheby 1870, pp. 225–226
- ^ Joerin 1909, p. 767
- ^ Bradbury 1909, p. 107
- ^ a b Angier 1910, p. 408
- ^ Brock 2008, p. 91
- ^ Salmon 1901, pp. 7–14
- ^ United States Herbarium 1890, p. 521
- ^ Wignall & Bowers 1993, p. 104
- ^ Foster & Johnson 2006, p. 190
- ^ Bostock & Alison 1832, p. 553
- ^ a b Cormack 1836, p. 58
- ^ Parr 1809, p. 383
- ^ a b Pliny 1856, p. 8
- ^ Berkeley 1744, p. 9
- ^ Pliny 1855, p. 290
- ^ a b c Cormack 1836, p. 50
- ^ Vitet 1778, p. 427
- ^ Chemist and Druggist 1889, p. 300
- ^ a b c d King, Felter & Llyod 1905, p. 617
- ^ Taylor 1902, p. 207
- ^ Whittaker 1893, p. 77
- ^ Imlay 1876, p. 514
- ^ Dobbell 1878, p. 315
- ^ a b Kinnicutt 1892, p. 514
- ^ Contrepois 2002, p. 211
- ^ Kinnicutt 1892, p. 515
- ^ Coblentz 1908
- ^ Farrar 1880, pp. 412–417
- ^ Farrar 1893, pp. 1–25
- ^ Pease 1862
- ^ Chenoweth 1945, p. 206
- ^ Commission of the European Communities 2001
- ^ Commission of the European Communities 2007
- ^ Heath and Safety Executive 2011
- ^ Creosote Council 2011
- ^ Ibach et al.
- ^ Seirogan 2011
References
[edit]- Schorlemmer, C. (1885). "The history of creosote, cedriret, and pittacal". Journal of the Society of Chemical Industry. 4. Society of Chemical Industry: 152–157.
- Thorpe, Sir Thomas Edward (1890). "Creosote". A Dictionary of Applied Chemistry. 1. Longmans, Green, and Co: 614–620.
- Allen, Alfred Henry (1910). "Creosote and Creosote oils". Allen's Commercial Organic Analysis. 3. P. Blakiston's Son & Co: 346–391.
- Roscoe, Henry Enfield; Schorlemmer, Carl (1888). "Creosote and Creosote oils". A Treatise on Chemistry: The Hydrocarbons and Their Derivatives or Organic Chemistry. 3:4. Appleton: 32–37.
- American Pharmaceutical Association (1895). "Creosote and Creosote oils". Proceedings of the American Pharmaceutical Association at the Annual Meeting. 43. The Association: 1073.
- Royal Chemical Society (1895). "Creosote and Creosote oils". Journal of the Chemical Society. 68:1. Royal Society of Chemistry: 294.
- Lee, Kwang-Guen; Lee, Sung-Eun; Takeoka, Gary R.; Kim, Jeong-Han; Park, Byeoung-Soo (July 2005). "Antioxidant activity and characterization of volatile constituents of beechwood creosote". Journal of the Science of Food and Agriculture. 85:9 (9). USDA: 1580–1586. doi:10.1002/jsfa.2156.
{{cite journal}}: CS1 maint: date and year (link) - Pharmaceutical Society of Great Britain (1898). "Creosotum". Pharmaceutical Journal: A Weekly Record of Pharmacy and Allied Sciences. 61. J. Churchill: 468.
- Abel, Ambrose; Smith, Elizur Goodrich (1857). The preservation of food: From the "Aus der natur" of Abel. Press of Case, Lockwood and company.
- Letheby, Henry (1870). On food: its varieties, chemical composition, nutritive value, comparative digestibility, physiological functions and uses, preparation, culinary treatment, preservation, adulteration, etc. Longmans, Green.
- United States Herbarium (1890). Contributions from the United States National Herbarium. Vol. 23. Smithsonian Institution Press. p. 521.
- Wignall, Brian; Bowers, Janice Emily (1993). Shrubs and trees of the Southwest deserts. Western National Parks Association. p. 104. ISBN 9781877856341.
- Foster, Stephen; Johnson, Rebecca L. (2006). Desk reference to nature's medicine. National Geographic Books. p. 190. ISBN 9780792236665.
- Bostock, John; Alison, William Pulteney (1832). The Cyclopædia of practical medicine: comprising treatises on the nature and treatment of diseases, materia medica and therapeutics, medical jurisprudence, etc. etc. Vol. 1. Sherwood, Gilbert, and Piper. p. 553.
- Cormack, Sir John Rose (1836). A treatise on the chemical, medicinal, and physiological properties of creosote: illustrated by experiments on the lower animals: with some considerations on the embalment of the Egyptians. Being the Harveian prize dissertation for 1836. J. Carfrae & Son.
- Parr, Bartholemew (1809). The London Medical Dictionary, including under distinct heads every branch of medecine. Vol. 1. J. Johnson.
- Berkeley, George (1744). Siris: a chain of philosophical reflexions and inquiries concerning the virtues of tar water, and divers other subjects connected together and arising one from another. Reprinted for W. Innys.
- Pliny (1855). Pliny's Natural History. Vol. 3. H. G. Bohn. ISBN 9780598910783.
- Pliny (1856). Pliny's Natural History. Vol. 5. G. Bell and sons.
- Vitet, Louis (1778). Pharmacopée de Lyon, ou exposition méthodique des médicaments simples et composés. Chez les Freres Perisse.
- Chemist and Druggist (1889). "Tar Water". Chemist and Druggist: The Newsweekly for Pharmacy. 35. Benn Brothers: 300.
- King, John; Felter, Harvey Wickes; Lloyd, John Uri (1905). "Creosote". King's American Dispensatory. 1. Ohio Valley Co: 616–617.
- Taylor, C.F. (1902). "Creosote". The Medical World. 20: 207.
- Whittaker, J.T. (1893). "Creasote in Tuberculosis Pulmonum". Transactions of the Association of American Physicians. 8. W.J. Dornan, inc: 77–90.
- Contrepois, Alain (2002). "The Clinician, Germs and Infectious Diseases: The Example of Charles Bouchard in Paris". Medical History. 46 (2): 197–220. doi:10.1017/s0025727300069088. PMC 1044495. PMID 12024808.
- Kinnicutt, Sir Francis P. (1892). "New outlooks in the prophylaxis and treatment of tuberculosis". Boston Medical and Surgical Journal. 126 (21): 513–518. doi:10.1056/NEJM189205261262101.
- Imlay, G. Anderson (1876). "New outlooks in the prophylaxis and treatment of tuberculosis". The Medical Times and Gazette. 2. J. & A. Churchill: 514.
- Dobbell, Horace (1878). "Carbolic acid and creosote". Annual Reports on Diseases of the Chest. 3. Smith: 315.
- Bernheim, Samuel (1901). La Tuberculose et la médication créosotée. Paris: Maloine.
- Coblentz, Virgil (1908). The newer remedies: including their synonyms, sources, tests, solubilities, incompatibilities, medicinal properties and doses as far as known, together with such proprietaries as have similar titles; a reference manual for physicians, pharmacists and students. The Apothecary Pub. Co.
- Engineering and Contracting (1912). "Wood Preserving Creosotes: Methods of Production, Properties, Quality, Price and Quantity Consumed in the United States". Engineering and Contracting. 38. Chicago: Myron C. Clark Publishing Co: 350–353.
- American Railway Bridge and Building Association (1914). "Wood Preserving Creosotes: Methods of Production, Properties, Quality, Price and Quantity Consumed in the United States". Proceedings of the Annual Convention of the American Railway, Bridge and Building Association. 23. Bretheren Publishing House: 287–288.
- Bateman, Ernest (1922). Coal-tar and water-gas tar creosotes. Govt. print. off.
- Angier, F.J. (1910). "The seasoning and preservative treatment of wood ties". Railway Age Gazette. 48: 408–411.
- Brock, William Hodson (2008). William Crookes and the commercialization of science. Ashgate Publishing, Ltd. ISBN 9780754663225.
- Price, Overton W.; Kellogg, R.S.; Cox, W.T. (1909). Forests of the United States: Their Use. Government printing office.
- Hodson, E.R. (1906). Rules and Regulations for the Grading of Lumber. Government printing office.
- Melber, Christine; Kielhorn, Janet; Mangelsdorf, Inge (2004). "COAL TAR CREOSOTE" (PDF). http://www.who.int/. World Heath Organization.
{{cite web}}: External link in|work= - Mueller, J.G.; Chapman, P.J.; Pritchard, P.H. (December 1989). "Action of a Fluoranthene-Utilizing Bacterial Community on Polycyclic Aromatic Hydrocarbon Components of Creosote". Applied and Environmental Microbiology. 55 (12). American Society for Microbiology: 3085–3090. doi:10.1128/AEM.55.12.3085-3090.1989. PMC 203227. PMID 16348069.
{{cite journal}}: CS1 maint: date and year (link) - Orr, Wilson L.; White, Curt M. (1990). Geochemistry of sulfur in fossil fuels. American Chemical Society. ISBN 9780841218048.
- Speight, J.G. (1994). The chemistry and technology of coal. CRC Press. ISBN 9780824792008.
- Mushrush, George C.; Speight, J.G. (1995). Petroleum products: instability and incompatibility. CRC Press. ISBN 9781560322979.
- Greenhow; E.J. (1965). Wood. Vol. 30. Tothill Press.
- Philips; H. Joshua (1891). Engineering chemistry: a practical treatise for the use of analytical chemists, engineers, ironmasters, iron founders, students, and others. C. Lockwood & son.
- Martin; Geoffrey (1913). Industrial and manufacturing chemistry: a practical treatise. Vol. 1. Appleton.
- Nelson; Thomas (1907). Nelson's encyclopaedia: everybody's book of reference. Vol. 3. Thomas Nelson.
- Noller; Carl Robert (1965). Chemistry of organic compounds. Saunders.
- Salmon, D.E. (1901). Relationship of bovine tuberculosis to public health. Government printing office.
- Seirogan (2011). "A Gift from the Forest". http://www.seirogan.co.jp/.
{{cite web}}: External link in|work= - Commission of the European Communities (2001). "COMMISSION DIRECTIVE 2001/90/EC". =http://eur-lex.europa.eu/.
{{cite web}}: External link in|work= - Commission of the European Communities (2007). "COMMISSION DIRECTIVE 76/769/EEC". =http://eur-lex.europa.eu/.
{{cite web}}: External link in|work= - Health and Safety Executive (2011). "Revocation of approvals for amateur creosote/coal tar creosote wood preservatives". =http://www.hse.gov.uk/.
{{cite web}}: External link in|work= - Creosote Council (2011). "Regulation". =http://creosotecouncil.org/.
{{cite web}}: External link in|work= - Ibach, Rebecca E.; Miller, Regis B. (2007). The Encyclopedia of Wood. Skyhorse Publishing Inc.
- Joerin, A.E. (December 1909). "The seasoning and preservative treatment of wood ties". Popular Mechanics. 48: 767.
{{cite journal}}: CS1 maint: date and year (link) - Bradbury, Robert H. (1909). "Increase in the use of wood preservatives indicates progress in wood preservation". Journal of the Franklin Institute. 168. Pergamon Press: 107. doi:10.1016/S0016-0032(09)90070-9.
- Farrar, J.N. (1880). "On the comparative value of sulphuric acid and creosote in the treatment of alveolar cavities". Annals of Anatomy and Surgery. 2: 412–418.
- Farrar, J.N. (1893). "Pulpless teeth; abscess; treatment, especially surgical treatment". Transactions of the New York Ondontological Society. J.P. Lippincott Company: 1–25.
- Pease, William A. (1862). "Arsenic, its application and use". British Journal of Dental Science. 5. Oxford Press: 417–426.
- Martin, Stanlisas (1862). "Solidified Creosote". British Journal of Dental Science. 5. Oxford Press: 290.
- Hunt, George McMonies; Garratt, George Alfred (1967). Wood preservation. McGraw-Hill.
- Voorhies, Glenn (June 1940). "Oil tar creosote for wood preservation". =http://ir.library.oregonstate.edu.
{{cite web}}: External link in|work= - Stimson, Earl (1914). "Report of the committee XVII on wood preservation". Proceedings of the Annual Convention of the American Railway, Bridge and Building Association. 15. Bretheren Publishing House: 625–633.
- Richardson, Barry A. (1993). Wood preservation. Taylor & Francis. ISBN 9780419174905.
- Encyclopaedia Britannica (1949). Encyclopaedia britannica: a new survey of universal knowledge. Vol. 21. Encyclopaedia Britannica.
- "Creosote: What you need to know" (PDF). http://www.losh.ucla.edu/. 2003.
{{cite web}}: External link in|work= - "Creosote (CASRN 8001-58-9)". http://www.epa.gov/. 1988.
{{cite web}}: External link in|work= - Wong O, Harris F (2005). "Retrospective cohort mortality study and nested case-control study of workers exposed to creosote at 11 wood-treating plants in the United States". J. Occup. Environ. Med. 47 (7): 683–97. doi:10.1097/01.jom.0000165016.71465.7a. PMID 16010195.
{{cite journal}}: Unknown parameter|month=ignored (help) - "Heating Fires in Residential Buildings" (PDF). http://www.usfa.dhs.gov/. 2006.
{{cite web}}: External link in|work= - Chenoweth, Walter Winfred (1945). How to preserve food. Houghton Mifflin company.