User:Blysse/sandbox
Superplasticizers (SPs), also known as high range water reducers, are additives used in making high strength concrete. Plasticizers are chemical compounds that enable the production of concrete with approximately 15% less water content. Superplasticizers allow reduction in water content by 30% or more. These additives are employed at the level of a few weight percent. Plasticizers and superplasticizers retard the curing of concrete.[1]
[2]Plasticizer us in concrete for workability, Strength, and Durability of concrete. Plasticizer in concrete for Water-reducing admixtures usually reduces the required water content for a concrete mixture by about 5 to 12 percent.
The use of water-reducing admixtures is defining as Type A in ASTM C 494. The WRA mainly affects the fresh properties of concrete.
SPs are used where well-dispersed particle suspension is required to improve the flow characteristics (rheology) of suspensions such as in concrete applications. Their addition to concrete or mortar allows the reduction of the water to cement ratio without negatively affecting the workability of the mixture, and enables the production of self-consolidating concrete and high performance concrete. They greatly improve the performance of the hardening fresh paste. The strength of concrete increases when the water to cement ratio decreases.[3]
The addition of SP in the truck during transit is a fairly modern development within the industry.
Mechanism
[edit]
Traditional plasticizers are lignosulphonates as their sodium salt.[4] Superplasticizers are synthetic polymers. Compounds used as superplasticizers include sulfonated naphthalene formaldehyde condensate, sulfonated melamine formaldehyde condensate, acetone formaldehyde condensate and polycarboxylate ethers. Cross-linked melamine- or naphthalene-sulfonates, referred to as PMS (polymelamine sulfonate) and PNS (polynaphthalene sulfonate), respectively, are illustrative. They are prepared by crosslinking of the sulfonated monomers using formaldehyde or by sulfonating the corresponding crosslinked polymer.[1][5]
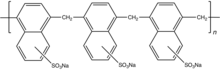
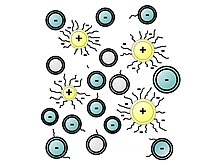
The polymers that serve as plasticizers exhibit surfactant properties. They are often ionomers. They function as dispersants to minimize particle segregation (gravel, coarse and fine sands). The negatively charged polymer backbone adsorbs on the positively charged colloidal particles. However, their working mechanisms lack a full understanding, revealing in certain cases cement-superplasticizer incompatibilities.[6]
See also
[edit]- Plasticizer
- Dispersant
- Rheology
- Workability
- Surfactant
- Particle aggregation (inverse process of)
- Peptization
- Suspension (chemistry)
References
[edit]- ^ a b Gerry Bye, Paul Livesey, Leslie Struble (2011). "Admixtures and Special Cements". Portland Cement: Third edition. doi:10.1680/pc.36116.185 (inactive 28 February 2022). ISBN 978-0-7277-3611-6.
{{cite book}}: CS1 maint: DOI inactive as of February 2022 (link) CS1 maint: multiple names: authors list (link) - ^ "Difference Between Plasticizer And Superplasticizer in Civil". CivilJungle. 2020-01-02. Retrieved 2020-08-14.
- ^ Houst, Yves F.; Bowen, Paul; Perche, Francois; Kauppi, Annika; Borget, Pascal; Galmiche, Laurent; Le Meins, Jean-Francois; Lafuma, Francoise; Flatt, Robert J.; Schober, Irene; et al. (2008). "Design and Function of Novel Superplasticizers for more Durable High performance Concrete (Superplast Project)". Cement and Concrete Research. 38 (10): 1197–1209. doi:10.1016/j.cemconres.2008.04.007.
- ^ a b R. Flatt, I. Schober (2012). "Superplasticizers and the rheology of concrete". In Nicolas Roussel (ed.). Understanding the Rheology of Concrete. Woodhead. ISBN 978-0-85709-028-7.
- ^ "A Review of Cement-superplasticizer interactions and their Models". Advances in Cement Research. 12 (4): 153–161. 2000. doi:10.1680/adcr.2000.12.4.153.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Ramachandran, V.S. (1995) Concrete Admixtures Handbook – Properties, Science, and Technology, 2nd Edition, William Andrew Publishing, ISBN 0-8155-1373-9 p. 121
