User:Armensouk/sandbox
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
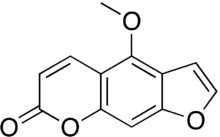
| Formula | C12H8O4 |
| Molar mass | 216.19 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bergapten (5-methoxypsoralen) is a psoralen (also known as furocoumarins) found in bergamot essential oil, in other citrus essential oils,[1] and in grapefruit juice along with various other plants like figs and parsley.[2] Bergapten is commonly found within the plant family Rutaceae. It is the chemical in bergamot oil that causes phototoxicity.[3] Bergapten-free bergamot essential oil or synthetics are now used in perfumery.
In 1834, 5-methoxypsoralen was the first furocoumarin to be isolated. It was isolated from Bergamot oil by Kalbrunner.[4] Furocoumarins are known for their photosensitizing effects, but bergapten can be a photo carcinogenic as well. The phototoxic components of bergapten are related to those of other furocoumarins like bergamottin.
In terms of structure and photosensitivity, there is considerable difference in photoreactivity to produce photosensitizing effects in skin between various furocoumarins.[4] While some can be very active, other furocoumarins like bergapten are weakly active. Still, linear furocoumarins like bergapten are generally more active than compounds with angular structures.[4]
Safety/Hazards
[edit]In 1931, Phyladelphy studied furocoumarins and observed how sunlight played a role in the reaction of furocoumarins with biological compounds. Kuske and his associate Mickelmann then established that it was furocoumarins that were the photosensitizing agents responsible for the development of phytophotodermatitis (Lime disease).[4]
Bergapten has since been found to have phototoxic effects, which causes skin to become photosensitive to the sun's rays, causing skin damage, redness, irritation and severe sun burn in some subjects. This has caused many of the essential oils with bergapten to be found with the bergapten removed for consumers who prefer that. The bergapten found within the oil can cause side effects like rashes, blisters and skin pigmentation changes potentially through the decrease of potassium levels in the body.[5] In terms of cosmetic products, the use of multiple essential oils is assumed to increase the risk of photoxicity proportionally. While steam inhalation and leave-on products have photoxicity cautions, ambient inhalation and wash-off products require no such warning.[6]
Bergapten is commonly found in earl grey tea through bergamot oil, and it has been implicated in side effects like muscle twitches, cramping in the hands and legs, and pressure in the eyes or blurry vision if too much earl grey tea is consumed.[5] These effects can be explained by bergapten's capacity as a potassium channel blocker. The reduction of potassium permeability at the nodes of Ranvier may lead to hyperexcitability of the axonal membrane and potassium current alterations, which can ultimately cause muscle cramps and fasciculations.[7]
5-methoxypsoralen can have a toxic or narcotic effect based on experiments with mice, rats, and fish that resulted in their death. Khadzai and others speculate that it is due to Bergapten's effect on the heart. 5-methoxypsoralen and other furocoumarins added to isolated rabbit heart lowered the arterial blood pressure, smooth muscle tonus and heart contractions while stimulating the rhythm of the heart.[4]
Bergapten, along with other furocoumarins, is able to induce a loss of template activity for RNA synthesis. Photosensitising effects of various furocoumarins has also been correlated with the inhibitory effect on template activity. 5-methoxypsoralen has also been implicated in having mutagenic effects, and it has the capacity of being a very potent agent for inducing chromosome aberrations. With a high enough concentration, complete mitotic inhibition was observed.[4]
Medical Usages
[edit]Bergapten serves to have the skin absorb more light, and pigmentary diseases like vitiligo (leukodermia) and psoriasis have treatments involving furocoumarins often in conjunction with sun exposure or solar radiation. In people who easily sunburn, furocoumarins can also increase the tolerance of skin to solar radiation.[4] Bergapten was shown to elicit certain skin reactions in order to even out pigmentation lightening for vitiligo patients depending on various factors like the susceptibility of the subject, the dosage, and the humidity, but the effects may be inconsistent.[5]
With psoriasis, bergapten has been valued as an oral photochemotherapy treatment for its efficacy and lack of phototoxic and drug-insensitive reactions. It operates as a photosensitizing drug that is as effective or, with high enough dosage, more effective than 8-methoxypsoralen in the clearance of psoriasis lesions.[8] It has been shown to be a valuable alternative to 8-methoxypsoralen due to the relative lack of side effects during treatment like erythmea, pruritus, and nausea. [9]
Bergapten has also been implicated as a potential prevention method for sunlight-related skin cancer. A particular study found that a tan gained with bergapten had less DNA damage in human subjects.[10] Bergapten has been shown to have anti-tumoral effects, like in its ability to induce the autophagic process in breast cancer cells. A particular study that this was possible through the up-regulation of PTEN gene expression in those breast cancer cells.[11]
Bergapten, alongside other furanocoumarins, has also been implicated in Cytochrome P450 inhibition.[12]
Synthesis
[edit]
Bergapten is a natural compound coming from plants like the common fig, but it can also be synthesized. Most syntheses of linear furanocoumarins involve starting with a central aromatic unit and adding two heterocyclic rings. Alternate routes of synthesis are desirable to avoid regiochemical problems and moderate yields. The synthesis described here involves Iodine as a removable group to insure regiochemical integrity and convergence.[13] As shown in the graphic, phloroglucinol (1) was the starting material. Mono-methylation was conducted followed by a reaction with ethyl propiolate in the presence of ZnCl2 to yield 7-hydroxy-5-methocycoumarin (3) with 68% yield. The 8-position of (3) was then protected by Iodine to avoid the formation of angular furanocoumarin. Product (4) in the graphic above is a result of that Iodine protection. Product 5 was a result of the allylation of product (4). Product (7) was the result of using osmium tetraoxide and sodium metraperiodate to oxidatively cleave the O-allyl derivative into the aldehyde (7) via a diol intermediate (6) (not shown in graphic). Cyclization of the aldehyde (7) using BF3-Et2O in tetra-n-butylammonium bromide was then conducted to construct the furan ring. The final step was then to remove the Iodine protective group via Pd(OAc)2 to ultimately result in Bergapten (9) with 90% yield. The bergapten was isolated as colorless needles with properties spectroscopially identical to the natural product.
A known use of bergapten is in the synthesis of Fraxinol.[14] The key reaction in this synthesis is the oxidation of the furan ring of visnagin and bergapten with chromic acid.[14]
References
[edit]- ^ Calvarano I.; Calvarano M.; Gionfriddo F.; Bovalo F.; Postorino E. (1995). "HPLC profile of citrus essential oils from different species and geographic origin". Essenze Derivati Agrumari. 65: 488–502.
- ^ Sakamaki N.; Nakazato M.; Matsumoto H.; Hagino K.; Hirata K.; Ushiyama H. (2008). "Contents of furanocoumarins in grapefruit juice and health foods". Journal of the Food Hygienic Society of Japan. 49 (4): 326–331. doi:10.3358/shokueishi.49.326. PMID 18787320.
- ^ Francesco Gionfriddo; Enrico Postorino; Giuseppe Calabrò (2004). "Elimination of Furocoumarins in Bergamot Peel Oil". Perfumer & Flavorist. 29.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ a b c d e f g Scott, Barry R (1976). "Molecular and genetic basis of furocoumarin reactions". Genetic Toxicology. 39 (1): 29–74. doi:10.1016/0165-1110(76)90012-9. PMID 13299.
- ^ a b c "Side effects of earl grey tea".
- ^ Tisserand, Robert (2014). "Essential Oil Profiles". Essential Oil Safety. second edition.
- ^ Finsterer, Josef (apr 2002). "Earl Grey tea intoxication". The Lancet. 359 (9316): 1484. doi:10.1016/S0140-6736(02)08436-2. PMID 11988248. S2CID 26873836.
{{cite journal}}: Check date values in:|date=(help) - ^ Honigsmann (Oct 1979). "5-Methoxypsoralen (Bergapten) in photochemotherapy of psoriasis". British Journal of Dermatology. 101 (4): 369–78. doi:10.1111/j.1365-2133.1979.tb00014.x. PMID 508604. S2CID 46371217.
- ^ Tanew, Adrian (February, 1988). "5-Methoxypsoralen (Bergapten) for photochemotherapy: Bioavailability, phototoxicity, and clinical efficacy in psoriasis of a new drug preparation". Journal of the American Academy of Dermatology. 18. doi:10.1016/S0190-9622(88)70048-1. PMID 3279089.
{{cite journal}}: Check date values in:|date=(help) - ^ Tisserand, Robert (2014). Essential Oil Safety. Churchill Livingstone.
- ^ De Amicis, Francesca (2015). "Bergapten drives autophagy through the up-regulation of PTEN expression in breast cancer cells". Molecular Cancer. 14.
- ^ Aldred, Elaine (2009). Haschek and Rousseaux's Handbook of Toxicologic Pathology (Third ed.). Churchill livingstone.
- ^ Oda, Kazuaki (June, 2005). "An Efficient Synthesis of Bergapten". Heterocycles. 65 (8): 1985–1988. doi:10.3987/COM-05-10451 – via ResearchGate.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Schönberg, Alexander; Badran, Nasry; Starkowsky, Nicolas A. (1955). "Furo-chromones and -Coumarins. XII. Synthesis of Fraxinol from Bergapten and of Baicalein from Visnagin". Journal of the American Chemical Society. 77 (20): 5390–5392. doi:10.1021/ja01625a055. ISSN 0002-7863.
