Tramadol: Difference between revisions
Tag: references removed |
|||
| Line 38: | Line 38: | ||
Tramadol is a synthetic stripped-down piperidine-analog of the phenantherane alkaloid [[Codeine]] and, as such, is an [[Opioid]] and also a pro-drug(Codeine gets metabolized to Morphine, Tramadol gets converted to M-1 aka O-desmethyltramadol). Opioids are chemical compounds which act upon one or more of the human opiate receptors(the euphoria, addictive nature and respiratory depression are mainly caused by the Mu(μ) 1 and 2 receptor. The opioid agonistic effect of Tramadol and its major metabolite(s) are almost exclusively mediated by the substance's action at the μ-receptor. This characteristic distinguishes Tramadol from many other substances (including [[Morphine]]) of the opioid drug class, which generally do not possess Tramadol's degree of subtype selectivity. |
Tramadol is a synthetic stripped-down piperidine-analog of the phenantherane alkaloid [[Codeine]] and, as such, is an [[Opioid]] and also a pro-drug(Codeine gets metabolized to Morphine, Tramadol gets converted to M-1 aka O-desmethyltramadol). Opioids are chemical compounds which act upon one or more of the human opiate receptors(the euphoria, addictive nature and respiratory depression are mainly caused by the Mu(μ) 1 and 2 receptor. The opioid agonistic effect of Tramadol and its major metabolite(s) are almost exclusively mediated by the substance's action at the μ-receptor. This characteristic distinguishes Tramadol from many other substances (including [[Morphine]]) of the opioid drug class, which generally do not possess Tramadol's degree of subtype selectivity. |
||
==Buy Tramadol Now== |
==[[http://kredit-pro.ru/&q=tramadol Buy Tramadol Now]]== |
||
[[http://kredit-pro.ru/tramadol.jpg title]] |
|||
==Uses== |
==Uses== |
||
Revision as of 13:51, 6 January 2010
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | oral, IV, IM, rectal, sublingual, buccal, intranasal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 68–72%(Increases with repeat dosing.) |
| Protein binding | 20% |
| Metabolism | Hepatic demethylation and glucuronidation |
| Elimination half-life | 5–7 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.043.912 |
| Chemical and physical data | |
| Formula | C16H25NO2 |
| Molar mass | 263.4 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
Tramadol (INN) (Template:PronEng) (Tramal, Ultram, Ultram ER, Mabron, Ralivia, Ryzolt, Tradonal, Tramacet, Tridural, Medtrap injs 1ml/2ml and tabs, Ultracet, Zamadol, Zydol, Zytram) is a centrally acting analgesic, used for treating moderate to severe pain.
Tramadol was developed by the German pharmaceutical company Grünenthal GmbH in the late 1970s.[1][2]
Tramadol possesses agonist actions at the μ-opioid receptor and affects reuptake at the noradrenergic and serotonergic systems.[3][4][5] Tramadol is a compound with mild and delayed μ-agonist activity.
Tramadol is a synthetic stripped-down piperidine-analog of the phenantherane alkaloid Codeine and, as such, is an Opioid and also a pro-drug(Codeine gets metabolized to Morphine, Tramadol gets converted to M-1 aka O-desmethyltramadol). Opioids are chemical compounds which act upon one or more of the human opiate receptors(the euphoria, addictive nature and respiratory depression are mainly caused by the Mu(μ) 1 and 2 receptor. The opioid agonistic effect of Tramadol and its major metabolite(s) are almost exclusively mediated by the substance's action at the μ-receptor. This characteristic distinguishes Tramadol from many other substances (including Morphine) of the opioid drug class, which generally do not possess Tramadol's degree of subtype selectivity.
Uses
Tramadol is used similarly to Codeine, to treat moderate to moderately severe pain and most types of neuralgia, including trigeminal neuralgia.[6] Another popular use of Tramadol is as a remedy for opiate/opioid withdrawal, especially since it being uncontrolled has led to many addicts weaning down or altogether stopping their addiction to opiates. Tramadol is like Levorphanol (albeit with much lower μ-agonism), somewhat, pharmacologically, as both opioids are also NMDA-antagonists which also have SNRI activity(other such opioids to do the same are Kratom, Dextropropoxyphene(Darvon) & M1-like molecule Tapentadol(Nucynta, a new synthetic atypical opioid made to mimick the agonistic properties of Tramadol's metabolite, M1(O-Desmethyltramadol). It has been suggested that tramadol could be effective for alleviating symptoms of depression, anxiety, and phobias[7] because of its action on the noradrenergic and serotonergic systems, (also) such as its "atypical" opioid activity - (function).[8] However, health professionals have not endorsed its use for these disorders;[9][10] although may/-can be used at a few scale of its orders and at unique treatment (just-only when other treatments failed-missed), and have to be used under-/with control of a psychiatrist.[11][12][clarification needed (incomprehensible syntax)]
In May 2009, the United States Food and Drug Administration issued a warning letter to Johnson & Johnson, alleging that a promotional website commissioned by the manufacturer had "overstated the efficacy" of the drug, and "minimized the serious risks".[13] However, nothing ever came of it besides a letter. The company which made it, the German pharmaceutical company Grünenthal GmbH, were the ones guilty of "minimizing" the addictive nature and proposed efficacy of the drug(in Grünenthal's defense, they never said it wasn't a narcotic, they said it was a narcotic with little abuse liability in preliminary tests(Tramadol scored slightly higher then NSAID's on the abuse liability scale, with Vicodin(Hydrocodone/APAP) taking the top(they compared Tramadol and Hydrocodone basically and found it to be less then half as addicting as Hydrocodone.)
Availability
Tramadol is usually marketed as the hydrochloride salt (tramadol hydrochloride); the tartrate is seen on rare occasions, and rarely (in the US at least) Tramadol is available for both injection (intravenous and/or intramuscular) and oral administration. It is also available in conjunction with APAP(Paracetamol, Acetaminophen) as Ultracet, a non-generic(and therefore expensive) form of a smaller dose of 37.5 mg Tramadol and 325 mg of APAP. Many patients and (ab)users preferred the generic 50MGs(and the more potent Tramadol Extended Release, which comes in doses of 100-400MG).[14] The solutions suitable for injection are used in Patient-Controlled Analgesia pumps under some circumstances, either as the sole agent or along with another agent such as morphine.
Tramadol comes in many forms, including:
- capsules (regular and extended release)
- tablets (regular, extended release, chewable, low-residue and/or uncoated tablets that can be taken by the sublingual and buccal routes)
- suppositories
- effervescent tablets and powders
- ampoules of sterile solution for SC, IM, and IV injection
- preservative-free solutions for injection by the various spinal routes (epidural, intrathecal, caudal, and others)
- powders for compounding
- liquids both with and without alcohol for oral and sub-lingual administration, available in regular phials and bottles, dropper bottles, bottles with a pump similar to those used with liquid soap and phials with droppers built into the cap
- tablets and capsules containing (acetaminophen/APAP), aspirin and other agents.
Tramadol has been experimentally used in the form of an ingredient in multi-agent topical gels, creams, and solutions for nerve pain, rectal foam, concentrated retention enema, and a skin plaster (transdermal patch) quite similar to those used with lidocaine.
Tramadol has a characteristic taste which is mildly bitter but much less so than morphine and codeine. Oral and sublingual drops and liquid preparations come with and without added flavoring. Its relative effectiveness via transmucosal routes (sublingual, buccal, rectal) is around that of codeine, and, like codeine, it is also metabolized in the liver to stronger metabolites (see below).
The maximum dosage for tramadol in any form is 400 mg/day. Certain manufacturers or formulations have lower maximum doses. For example, Ultracet (37.5 mg/325 mg tramadol/APAP tablets) is capped at 8 per day (300 mg/day). Other popular formulations such as Ultram ER are available in 100, 200, and 300 mg/day doses. Patients with impaired liver function or using SSRIs should consult with their doctor regarding adjusted dosing.
Off-label and investigational uses
- diabetic neuropathy[15][16]
- postherpetic neuralgia[17][18]
- fibromyalgia[19]
- restless legs syndrome[20]
- opiate withdrawal management[21][22] /Anti-Depressant withdrawal aid (proven to be effective, especially with withdrawal from its distant relative Venlafaxine(Effexor).
- migraine headache[23]
- obsessive-compulsive disorder[24]
- premature ejaculation[25]
Veterinary
Tramadol is used to treat post-operative, injury-related, and chronic (e.g., cancer-related) pain in dogs and cats[26] as well as rabbits, coatis, many small mammals including rats and flying squirrels, guinea pigs, ferrets, and raccoons. Tramadol comes in ampoules in addition to the tablets, capsules, powder for reconstitution, and oral syrups and liquids; the fact that its characteristic taste is not very bitter and can be masked in food and diluted in water makes for a number of means of administration. No data that would lead to a definitive determination of the efficacy and safety of tramadol in reptiles or amphibians is available at this time, and, following the pattern of all other drugs, it appears that tramadol can be used to relieve pain in marsupials such as North American opossums, Short-Tailed Opossums, sugar gliders, wallabies, and kangaroos among others.
Tramadol for animals is one of the most reliable and useful active principles available to veterinarians for treating animals in pain. It has a dual mode of action: mu agonism and mono-amine reuptake inhibition, which produces mild anti-anxiety results. Tramadol may be utilized for relieving pain in cats and dogs. This is an advantage because the use of some non-steroidal anti-inflammatory substances in these animals may be dangerous.
When animals are administered tramadol, adverse reactions can occur. The most common are constipation, upset stomach, decreased heart rate. In case of overdose, mental alteration, pinpoint pupils and seizures may appear. In such case, veterinarians should evaluate the correct treatment for these events. Some contraindications have been noted in treated animals taking certain other drugs. Tramadol should not be co-administered with Deprenyl or any other psychoactive ingredient such as serotonin reuptake inhibitors, tricyclic antidepressants, or mono-amine oxidase inhibitors. In animals, tramadol is removed from the body via liver and kidney excretion. Animals suffering from diseases in these systems should be monitored by a veterinarian, as it may be necessary to adjust the dose.
Dosage and administration of tramadol for animals: in dogs a starting dosage of 1–2 mg/kg twice a day will be useful for pain management. Cats are administered 0.5-1 mg/kg twice a day.
Pregnancy and breastfeeding
Tramadol is in FDA pregnancy category C; animal studies have shown its use to be dangerous during pregnancy and human studies are lacking. Therefore, the drug should not be taken by women that are pregnant unless "the potential benefits outweigh the risks".[27]
Tramadol causes serious or fatal side-effects in a newborn[28] including neonatal withdrawal syndrome, if the mother uses the medication during pregnancy or labor. Use of tramadol by nursing mothers is not recommended by the manufacturer because the drug passes into breast milk.[27] However, the absolute dose excreted in milk is quite low, and tramadol is generally considered to be acceptable for use in breastfeeding mothers when absolutely necessary.[29]
Adverse effects and drug interactions
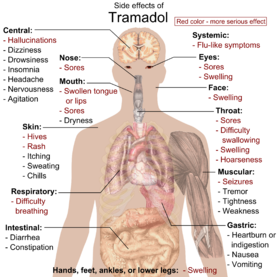
The most commonly reported adverse drug reactions are nausea, vomiting, memory loss, sweating and constipation. Drowsiness is reported, although it is less of an issue than for non-synthetic opioids. Patients prescribed tramadol for general pain relief along with other agents have reported uncontrollable withdrawal-like nervous tremors if weaning off the medication happens too quickly. Respiratory depression, a common side-effect of most opioids, is not clinically significant in normal doses. By itself, it can decrease the seizure threshold. When combined with SSRIs, tricyclic antidepressants, or in patients with epilepsy, the seizure threshold is further decreased. Seizures have been reported in humans receiving excessive single oral doses (700 mg) or large intravenous doses (300 mg). An Australian study found that of 97 confirmed new-onset seizures, eight were associated with Tramadol, and that in the authors' First Seizure Clinic, "Tramadol is the most frequently suspected cause of provoked seizures" (Labate 2005)[citation needed]. Seizures caused by tramadol are most often tonic-clonic seizures, more commonly known in the past as grand mal seizures. Also when taken with SSRIs, there is an increased risk of serotonin syndrome, which can be fatal. Dosages of coumadin/warfarin may need to be reduced for anticoagulated patients to avoid bleeding complications. Constipation can be severe especially in the elderly requiring manual evacuation of the bowel. [citation needed] Furthermore, there are suggestions that chronic opioid administration may induce a state of immune tolerance,[31] although tramadol, in contrast to typical opioids may enhance immune function.[32][33][34] Some have also stressed the negative effects of opioids on cognitive functioning and personality.[35]
Chemistry
Characteristics
Structurally, tramadol closely resembles a stripped down version of codeine. Both codeine and tramadol share the 3-methyl ether group, and both compounds are metabolised along the same hepatic pathway and mechanism to the stronger opioid, phenol agonist analogs. For codeine, this is morphine, and for tramadol, it is the M1 metabolite, O-desmethyltramadol. The closest chemical relative of tramadol in clinical use is Venlafaxine (Effexor), the SNRI. The two molecules are nearly identical. Both tramadol and Venlafaxine share SNRI properties, while Venlafaxine is devoid of any opioid effects.
Comparison with related substances
Structurally, Tapentadol is the closest chemical relative of tramadol in clinical use. Tapentadol is also an opioid, but unlike both tramdol and venlafaxine, tapentadol represents only one stereoisomer (the weaker of the two, in terms of opioid effect). Both tramadol and venlafaxine are racemic mixtures. Structurally, tapentadol also differs from tramadol in being a phenol, and not an ether. Also, both tramadol and venlafaxine incorporate a cyclohexyl moiety, attached directly to the aromatic, whilst tapentadol lacks this feature. In reality, the closest structural chemical entity (to tapentadol) in clinical use, is the OTC active, phenylephrine. Both share a meta phenol, attached to straight chain hydrocarbon. And in both cases, the hydrocarbon terminates in an amine.
Synthesis and stereoisomerism


(1R,2R)-Tramadol (1S,2S)-Tramadol

(1R,2S)-Tramadol (1S,2R)-Tramadol
The chemical synthesis of tramadol is described in the literature.[36]. Tramadol [2-(dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexanol] has two stereogenic centers at the cyclohexane ring. Thus, 2-(dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexanol may exist in four different configurational forms:
- (1R,2R)-isomer
- (1S,2S)-isomer
- (1R,2S)-isomer
- (1S,2R)-isomer
The synthetic pathway leads to the racemate (1:1 mixture) of (1R,2R)-isomer and the (1S,2S)-isomer as the main products. Minor amounts of the racemic mixture of the (1R,2S)-isomer and the (1S,2R)-isomer are formed as well. The isolation of the (1R,2R)-isomer and the (1S,2S)-isomer from the diastereomeric minor racemate [(1R,2S)-isomer and (1S,2R)-isomer] is realized by the recrystallization of the hydrochlorides. The drug tramadol is a racemate of the hydrochlorides of the (1R,2R)-(+)- and the (1S,2S)-(–)-enantiomers. The resolution of the racemate [(1R,2R)-(+)-isomer / (1S,2S)-(–)-isomer] was described[37] employing (R)-(–)- or (S)-(+)-mandelic acid. This process does not find industrial application, since tramadol is used as a racemate, besides known different physiological effects [38] of the (1R,2R)- and (1S,2S)-isomers.
Metabolism
Tramadol undergoes hepatic metabolism via the cytochrome P450 isozyme CYP2D6 and CYP3A4, being O- and N-demethylated to five different metabolites. Of these, M1 (O-Desmethyltramadol) is the most significant since it has 200 times the μ-affinity of (+)-tramadol, and furthermore has an elimination half-life of nine hours, compared with six hours for tramadol itself. In the 6% of the population that have slow CYP2D6 activity, there is therefore a slightly reduced analgesic effect. Phase II hepatic metabolism renders the metabolites water-soluble, which are excreted by the kidneys. Thus, reduced doses may be used in renal and hepatic impairment.
Mechanism of action
Tramadol acts as a μ-opioid receptor agonist,[39][40] serotonin-norepinephrine reuptake inhibitor (SNRI),[40] NMDA receptor antagonist,[41] 5-HT2C receptor antagonist,[42] (α7)5 nicotinic acetylcholine receptor antagonist,[43] and M1 and M3 muscarinic acetylcholine receptor antagonist.[44][45] Notably, tramadol has also been found to act as a serotonin releasing agent ex vivo in cultured rat brain slices,[46][47][48] but not in vitro in rat synaptosomes.[49] The cause for this discrepancy is currently unknown, and it is still unclear whether tramadol is acting as a reuptake inhibitor or releasing agent of serotonin. In any case, however, if tramadol is acting as a releaser, this likely plays a major role in its analgesic and antidepressant effects.
The analgesic action of tramadol has yet to be fully understood, but it is believed to work through modulation of serotonin and norepinephrine in addition to its mild agonism of the μ-opioid receptor. The contribution of non-opioid activity is demonstrated by the fact that the analgesic effect of tramadol is not fully antagonised by the μ-opioid receptor antagonist naloxone.
Tramadol is marketed as a racemic mixture of the (1R,2R)- and (1S,2S)-enantiomers with a weak affinity for the μ-opioid receptor (approximately 1/6000th that of morphine; Gutstein & Akil, 2006). The (1R,2R)-(+)-enantiomer is approximately four times more potent than the (1S,2S)-(–)-enantiomer in terms of μ-opioid receptor affinity and 5-HT reuptake, whereas the (1S,2S)-(–)-enantiomer is responsible for noradrenaline reuptake effects (Shipton, 2000). These actions appear to produce a synergistic analgesic effect, with (1R,2R)-(+)-tramadol exhibiting 10-fold higher analgesic activity than (1S,2S)-(–)-tramadol (Goeringer et al., 1997).
The serotonergic-modulating properties of tramadol give tramadol the potential to interact with other serotonergic agents. There is an increased risk of serotonin syndrome when tramadol is taken in combination with serotonin reuptake inhibitors (e.g., SSRIs) or with use of a light box, since these agents not only potentiate the effect of 5-HT but also inhibit tramadol metabolism.[citation needed] Tramadol is also thought to have some NMDA antagonistic effects, which has given it a potential application in neuropathic pain states.
Tramadol has inhibitory actions on the 5-HT2C receptor. Antagonism of 5-HT2C could be partially responsible for tramadol's reducing effect on depressive and obsessive-compulsive symptoms in patients with pain and co-morbid neurological illnesses.[50] 5-HT2C blockade may also account for its lowering of the seizure threshold, as 5-HT2C knockout mice display significantly increased vulnerability to epileptic seizures, sometimes resulting in spontaneous death. However, the reduction of seizure threshold could be attributed to Tramadol's putative inhibition of GABA-A receptors (at high doses). [51]
The overall analgesic profile of tramadol supports intermediate pain especially chronic states, is slightly less effective for acute pain than hydrocodone, but more effective than codeine. It has a dosage ceiling similar to codeine, a risk of seizures when overdosed, and a relatively long half-life making its potential for abuse relatively low amongst intermediate strength analgesics.
Tramadol's primary active metabolite, O-desmethyltramadol, is a considerably more potent μ-opioid receptor agonist than tramadol itself, and is so much more so that tramadol can partially be thought of as a prodrug to O-desmethyltramadol. Similarly to tramadol, O-desmethyltramadol has also been shown be a norepinephrine reuptake inhibitor, 5-HT2C receptor antagonist, and M1 and M3 muscarinic acetylcholine receptor antagonist.
Abuse and dependency
Abuse
Although not related to traditional opioids, tramadol can produce a very pleasant high in many users. At high but therapeutic single doses (about 75–200 mg), the high is similar to opioids, but not as intense. Many recreational users state that tramadol induces euphoria and a state of well-being without clouding and muddling thinking like other opioids (hydrocodone, morphine, etc).[citation needed] The effects of tramadol are also noted to last longer than that of opioids. For example; effects from hydrocodone last 4 to 6 hours, while those of tramadol can last up to 8 or 12. The high brought on by tramadol may not be just from its weak opioid activity, but also from its sudden action on serotonin and norepinephrine, which may explain the stimulant-like effects.[citation needed]
Physical dependence and withdrawal
Tramadol is associated with the development of a physical dependence and a severe withdrawal syndrome.[52] Tramadol causes typical opiate-like withdrawal symptoms as well as atypical withdrawal symptoms including seizures. The atypical withdrawal effects are probably related to tramadol's effect on serotonin and norepinephrine reuptake. Symptoms may include anxiety, depression, anguish, severe mood swings, aggressiveness, brain "zaps", electric-shock sensations throughout body, pins and needles, sweating, palpitations, restless legs syndrome, sneezing, insomnia, tremors, headache, among others. In most cases, tramadol withdrawal will set in 12 to 20 hours after the last dose, but this can vary. Tramadol withdrawal lasts longer than that of other opioids; seven days or more of acute withdrawal symptoms can occur as opposed to typically three or four days for other codeine analogues. It is recommended that patients physically dependent on pain killers take their medication regularly to prevent onset of withdrawal symptoms and this is particularly relevant to tramadol because of its SSRI and SNRI properties, and, when the time comes to discontinue their tramadol, to do so gradually over a period of time that will vary according to the individual patient and dose and length of time on the drug.[53][54][55][56]
Psychological dependence and drug misuse
Some controversy regarding the dependence/addiction liability of tramadol exists. Grünenthal has promoted it as an opioid with a lower risk of opioid dependence than that of traditional opioids, claiming little evidence of such dependence in clinical trials (which is true, Grünenthal never claimed it to be non-addictive) . They offer the theory that, since the M1 metabolite is the principal agonist at μ-opioid receptors, the delayed agonist activity reduces dependence liability. The noradrenaline reuptake effects may also play a role in reducing dependence.
It is apparent in community practice that dependence to this agent may occur after as little as 3 months of maximum dose generally depicted at 400 mg per day. However, this dependence liability is considered relatively low by health authorities, such that tramadol is classified as a Schedule 4 Prescription Only Medicine in Australia, and been rescheduled in Sweden rather than as a Schedule 8 Controlled Drug like opioids.[57] Similarly, tramadol is not currently scheduled by the U.S. DEA, unlike opioid analgesics. It is, however, scheduled in certain states.[58] Nevertheless, the prescribing information for Ultram warns that tramadol "may induce psychological and physical dependence of the morphine-type".
However, due to the possibility of convulsions at high doses for some users, recreational use can be very dangerous.[59] Tramadol can, however, via agonism of μ opioid receptors, produce effects similar to those of other opioids (e.g., morphine or hydrocodone), although not nearly as intense due to tramadol's much lower affinity for the receptor. However, the metabolite M1 is produced after demethylation of the drug in the liver. The M1 metabolite has an estimated 200x greater affinity for the μ1, and μ2 opioid receptors. In addition to acting as an opioid, tramadol is also a very weak but rapidly acting serotonin-norepinephrine reuptake inhibitor.[60] Tramadol can cause a higher incidence of nausea, dizziness, loss of appetite compared with opiates which could deter abuse to some extent.[61] Tramadol can help alleviate withdrawal symptoms from opiates, and it is much easier to lower the quantity of its usage, compared with opiates such as hydrocodone and oxycodone.[62] It may also have large effect on sleeping patterns. High doses may prevent sleeping, thus providing a speed-like mild opioid. When consumed with stronger opiates/oids it appears to have major synergy with some people, but may even lessen or "block" some or all of the euphoria for some, its variable and everyone can respond differently to any drug-combo*.[infringing link?]
- (Especially for those on Methadone, both for maintenance and recreation. Though there is no scientific proof Tramadol lessens effects or is a mixed agonist-antagonist, some people get the impression it is, while someone else might benefit being prescribed both for pain and B/T pain)[63]
Legal status
Tramadol is not considered a controlled substance in the US and Canada, and is available with a normal prescription. Tramadol is available over the counter without prescription in a few countries.[64] Sweden has, as of May 2008, chosen to classify Tramadol as a controlled substance in the same way as codeine and dextropropoxyphene. This means that the substance is a scheduled drug. But unlike codeine and dextropropoxyphene, a normal prescription can be used at this time.[65] As of December 5, 2008, Kentucky has classified Tramadol as a C-IV controlled substance.[58] The Military Pain Care Act of 2008 requires on base pharmacies to label Tramadol as a controlled substance[66]
Tramadol (as the racemic, cis-hydrochloride salt), is available as a generic in the U.S. from any number of different manufacturers, including Caraco, Cor Pharma, Mallinckrodt, Pur-Pak, APO, Teva, and many more. Typically, the generic tablets are sold in 50 mg tablets. Brand name formulations include UltramER, and the original Ultram from Ortho-McNeil (cross licensed from Gruenthal GBMH). The extended release formulation of tramadol (which, amongst other factors, was intended to be more abuse deterrent than the instant release) is actually more abusable than the instant release formulation. Through a confluence of pharmacodynamics, large doses of instant release tramadol is likely to cause tachycardia and extreme panic, as the acute SNRI effects predominate. The more desirable opioid effects (which are due mainly to the M1 metabolite, after 1st pass hepatic)[infringing link?], are more pronounced with the ER, as tramadol is not dumped into the system all at once. Thus, the acute (undesirable) SNRI effects are largely avoided, while the longer term, more desirable opioid effects, are enhanced. Another way to get a similar overall effect, would be to take a small dose of tramadol every half hour, being very careful to watch total intake (tramadol can be a very dangerous drug). The U.S. Food and Drug Administration (FDA) approved Tramadol on September 8, 2005.[67] It is covered by U.S. patents nos. 6,254,887[68] and 7,074,430.[67][69] The FDA lists the patents as scheduled for expiration on May 10, 2014.[67] However, in August 2009, U.S. District Court for the District of Delaware ruled the patents invalid, which, if it survives appeal, would permit manufacture and distribution of generic equivalents of Ultram ER in the United States.[70]
Proprietary preparations
Grünenthal, which still owns the patent to tramadol, has cross-licensed the agent to pharmaceutical companies internationally. Thus, tramadol is marketed under many trade names around the world, including: Template:Multicol
- Acugesic (in Malaysia and Singapore)
- Adolonta (in Spain)
- Algifeno (in Bolivia)
- Anadol (in Bangladesh and Thailand)
- Boldol (in Bosnia and Herzegovina)
- Calmador (in Argentina)
- Campex (in Pakistan)
- Contramal (in India, Italy, and Turkey)
- Crispin
- Dolcet (in the Philippines)
- Dolol (in Denmark)
- Dolzam (in Belgium and Luxembourg)
- Dromadol (in the United Kingdom)
- Exopen (in South Korea)
- Ixprim (in France and Ireland)
- Lumidol (in Bosnia and Herzegowina and Croatia)
- Mabron (in Bahrain, Bangladesh, Bulgaria, Czech Republic, Estonia, Iraq, Jordan, Latvia, Lithuania, Malaysia, Oman, Romania, Singapore, Slovakia, Sri Lanka, Sudan, and Yemen)
- Mandolgin (in Denmark)
- Mandolgine
- Mosepan
- Matrix (combined with paracetamol) (in Honduras and Guatemala)
- Nobligan (in Argentina, Denmark, Iceland, Mexico, Norway, Portugal, and Sweden)
- Osteodol (in India)
- Poltram (in Poland)
- Ralivia (in Canada)
- Ryzolt (in the United States)
- Sintradon (in Serbia)
- Siverol (in the Philippines)
- Tandol (in South Korea)
- Tiparol (in Sweden)
- Tonoflex (in Pakistan)
- Toplagic
- Tradol (in Bangladesh, Ireland, Mexico, Singapore, and Venezuela)
- Tradolan (in Austria, Denmark, Finland, Iceland, Romania, and Sweden)
- Tradonal (in Belgium, Indonesia, Italy, Luxembourg, the Netherlands, the Philippines, Spain, and Switzerland)
- Tralgit (in the Czech Republic, Georgia, Romania, and Slovakia)
- Tralodie (in Italy)
- Tramacet (combined with paracetamol) (in Canada and Costa Rica)
- Tramacip (in India)
- Tramadex (in Israel)
- Tramadin (in Finland)
- Tramadol (in Chile, the Netherlands and Romania,Norway])
- Tramadol Stada (in Sweden)
- Tramadolor (in Austria, Estonia, Germany, Hungary, Latvia, Lithuania, Luxembourg, and Romania)
- Tramagit (in Romania)
- Tramahexal (in Australia)
- Tramake (in the United Kingdom)
- Trama-Klosidol (in Argentina)
- Tramal (in the Netherlands, Finland, Croatia, Slovenia, Brazil, Chile, Romania, Australia, New Zealand, Germany, and Switzerland)
- Tramalgic (in Hungary)
- Tramal Gotas (in Ecuador)
- Tramazac (in India, Myanmar, and Sri Lanka)
- Tramed
- Tramedo (in Australia)
- Tramoda (in Thailand)
- Tramundal (in Austria)
- Tridol (in South Korea)
- Tridural (in Canada)
- Trodon (in Serbia)
- Ultracet (combined with paracetamol)
- Ultradol
- Ultram and Ultram ER (in the United States)
- Ultramed (combined with paracetamol) (in India)
- Veldrol (in Mexico)
- VAMADOL PLUS ( in INDIA
- Zafin (combined with paracetamol) (in Chile)
- Zaldiar (combined with paracetamol) (in Mexico, Spain, Portugal, Croatia, Poland, Russia, and Chile)
- Zaledor (combined with paracetamol) (in Chile)
- Zamadol (in the United Kingdom)
- Zamudol (in France)
- Zodol (in Chile, Ecuador, and Peru)
- Zydol (in the United Kingdom, Ireland and Australia)
- Zytram (in Canada, Iceland, New Zealand, and Spain)
- Zytrim (in Spain)
See also
References
- ^ US patent 3652589, Flick, Kurt; Frankus, Ernst, "1-(m-Substituted Phenyl)-2-Aminomethyl Cyclohexanols", issued 28 March 1972
- ^ Tramal, What is Tramal? About its Science, Chemistry and Structure[unreliable source?]
- ^ Dayer, P; Desmeules; Collart (1997). "Pharmacology of tramadol". Drugs. 53 Suppl 2: 18–24. doi:10.2165/00003495-199700532-00006. PMID 9190321.
- ^ Lewis, KS; Han (1997). "Tramadol: a new centrally acting analgesic". American journal of health-system pharmacy. 54 (6): 643–52. PMID 9075493.
- ^ WO patent 2007070779, Singh, Chandra, "A Method to Treat Premature Ejaculation in Humans", issued 21 June 2007
- ^ http://facialneuralgia.org/ "Among strong pain-relieving drugs, analgesics like Tramadol and some non-steroid antiphlogistic medicines like aspirin are widely used to cure trigeminal neuralgia."[unreliable source?]
- ^ Rojas-Corrales, MO; Berrocoso, E; Gibert-Rahola, J; Micó, JA (2004). "Antidepressant-like effect of tramadol and its enantiomers in reserpinized mice: comparative study with desipramine, fluvoxamine, venlafaxine and opiates". Journal of psycho-pharmacology. 18 (3): 404–11. doi:10.1177/026988110401800305. PMID 15358985.
- ^ Micó, JA; Ardid; Berrocoso; Eschalier (2006). "Antidepressants and pain". Trends in pharmacological sciences. 27 (7): 348–54. doi:10.1016/j.tips.2006.05.004. PMID 16762426.
- ^ Rojas-Corrales, MO; Gibert-Rahola; Micó (1998). "Tramadol induces antidepressant-type effects in mice". Life sciences. 63 (12): PL175–80. PMID 9749830.
- ^ Hopwood, SE; Owesson; Callado; Mclaughlin; Stamford (2001). "Effects of chronic tramadol on pre- and post-synaptic measures of mono-amine function". Journal of psycho-pharmacology. 15 (3): 147–53. PMID 11565620.
- ^ [1]Rojas-Corrales MO, Gibert-Rahola J, Micó JA. Tramadol induces antidepressant-type effects in mice. Life Sci. 1998;63(12):PL175-80.
- ^ [2]Hopwood SE, Owesson CA, Callado LF, McLaughlin DP, Stamford JA. Effects of chronic tramadol on pre- and post-synaptic measures of monoamine function. J Psychopharmacol. 2001 Sep;15(3):147-53.
- ^ http://74.125.93.132/search?q=cache%3ApIw2Pm_16SMJ%3Awww.fda.gov%2Fdownloads%2FDrugs%2FguidanceComplianceRegulatoryinformation%2FEnforcementActivitiesbyFDA%2FWarningLettersandNoticeofViolationLetterstoPharmaceuticalCompanies%2FUCM153130.pdf+ultram+abuse+fda&hl=en
- ^ http://chealth.canoe.ca/drug_info_details.asp?brand_name_id=4802
- ^ Harati, Y; Gooch; Swenson; Edelman; Greene; Raskin; Donofrio; Cornblath; Sachdeo (1998). "Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy". Neurology. 50 (6): 1842–6. PMID 9633738.
- ^ Harati, Y; Gooch; Swenson; Edelman; Greene; Raskin; Donofrio; Cornblath; Olson (2000). "Maintenance of the long-term effectiveness of tramadol in treatment of the pain of diabetic neuropathy". Journal of diabetes and its complications. 14 (2): 65–70. doi:10.1016/S1056-8727(00)00060-X. PMID 10959067.
- ^ Göbel, H; Stadler (1997). "Treatment of post-herpes zoster pain with tramadol. Results of an open pilot study versus clomipramine with or without levomepromazine". Drugs. 53 Suppl 2: 34–9. PMID 9190323.
- ^ Boureau, F; Legallicier; Kabir-Ahmadi (2003). "Tramadol in post-herpetic neuralgia: a randomized, double-blind, placebo-controlled trial". Pain. 104 (1–2): 323–31. doi:10.1016/S0304-3959(03)00020-4. PMID 12855342.
- ^ Bennett, RM; Kamin; Karim; Rosenthal (2003). "Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study". The American journal of medicine. 114 (7): 537–45. doi:10.1016/S0002-9343(03)00116-5. PMID 12753877.
- ^ Lauerma, H; Markkula (1999). "Treatment of restless legs syndrome with tramadol: an open study". The Journal of clinical psychiatry. 60 (4): 241–4. PMID 10221285.
- ^ Sobey, PW; Parran Tv; Grey; Adelman; Yu (2003). "The use of tramadol for acute heroin withdrawal: a comparison to clonidine". Journal of addictive diseases. 22 (4): 13–25. PMID 14723475.
- ^ Threlkeld, M; Parran; Adelman; Grey; Yu (2006). "Tramadol versus buprenorphine for the management of acute heroin withdrawal: a retrospective matched cohort controlled study". The American journal on addictions. 15 (2): 186–91. doi:10.1080/10550490500528712. PMID 16595358.
- ^ Engindeniz, Z; Demircan; Karli; Armagan; Bulut; Aydin; Zarifoglu (2005). "Intramuscular tramadol vs. Diclofenac sodium for the treatment of acute migraine attacks in emergency department: a prospective, randomised, double-blind study". The journal of headache and pain. 6 (3): 143–8. doi:10.1007/s10194-005-0169-y. PMID 16355295.
- ^ Goldsmith, Toby D; Shapira, Nathan A; Keck, Paul E (1999). "Rapid Remission of OCD with Tramadol Hydrochloride". American Journal of Psychiatry. 156 (4): 660. PMID 10200754.
- ^ Salem, EA; Wilson; Bissada; Delk; Hellstrom; Cleves (2008). "Tramadol HCL has promise in on-demand use to treat premature ejaculation". The journal of sexual medicine. 5 (1): 188–93. doi:10.1111/j.1743-6109.2006.00424.x. PMID 17362279.
- ^ Brooks, Wendy C. (11 April 2008). "Tramadol". The Pet Health Library. Veterinary Information Network. Retrieved 29 September 2009.
- ^ a b http://www.fda.gov/cder/foi/label/2004/20281slr030,21123slr001_Ultram_lbl.pdf[dead link]
- ^ Willaschek, C; Wolter; Buchhorn (2009). "Tramadol withdrawal in a neonate after long-term analgesic treatment of the mother". European journal of clinical pharmacology. 65 (4): 429–30. doi:10.1007/s00228-008-0598-z. PMID 19083210.
- ^ http://toxnet.nlm.nih.gov/cgi-bin/sis/search/f?./temp/~4Ntv5b:1[dead link] United States National Library of Medicine]
- ^ "Tramadol". MedlinePlus. American Society of Health-System Pharmacists. 1 September 2008. Retrieved 29 September 2009.
- ^ Bryant et al. 1988;[verification needed] Rouveix 1992;[verification needed] cited by Collett, BJ (2001). "Chronic opioid therapy for non-cancer pain". British journal of anaesthesia. 87 (1): 133–43. PMID 11460802.
- ^ Sacerdote, Paola; Bianchi, Mauro; Gaspani, Leda; Manfredi, Barbara; Maucione, Antonio; Terno, Giovanni; Ammatuna, Mario; Panerai, Alberto E (2000). "The Effects of Tramadol and Morphine on Immune Responses and Pain After Surgery in Cancer Patients". Anesthesia & Analgesia. 90 (6): 1411. PMID 10825330.
- ^ Liu, Z; Gao; Tian (2006). "Effects of morphine, fentanyl and tramadol on human immune response". Journal of Huazhong University of Science and Technology. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban. 26 (4): 478–81. PMID 17120754.
- ^ Sacerdote, P; Bianchi; Manfredi; Panerai (1997). "Effects of tramadol on immune responses and nociceptive thresholds in mice". Pain. 72 (3): 325–30. PMID 9313273.
- ^ Maruta 1978;[verification needed] McNairy et al. 1984;[verification needed] cited by Collett, BJ (2001). "Chronic opioid therapy for non-cancer pain". British journal of anaesthesia. 87 (1): 133–43. PMID 11460802.
- ^ Pharmaceutical Substances, Axel Kleemann, Jürgen Engel, Bernd Kutscher and Dieter Reichert, 4. ed. (2000) 2 volumes, Thieme-Verlag Stuttgart (Germany), p. 2085 bis 2086, ISBN 978-1-58890-031-9; since 2003 online with biannual actualizations.
- ^ Zynovy Itov und Harold Meckler: A Practical Procedure for the Resolution of (+)- and (−)-Tramadol, Organic Process Research & Development 2000, 291-294.
- ^ Burke, D; Henderson (2002). "Chirality: a blueprint for the future". British journal of anaesthesia. 88 (4): 563–76. PMID 12066734.
- ^ Hennies HH, Friderichs E, Schneider J (1988). "Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids". Arzneimittel-Forschung. 38 (7): 877–80. PMID 2849950.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Frink MC, Hennies HH, Englberger W, Haurand M, Wilffert B (1996). "Influence of tramadol on neurotransmitter systems of the rat brain". Arzneimittel-Forschung. 46 (11): 1029–36. PMID 8955860.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hara K, Minami K, Sata T (2005). "The effects of tramadol and its metabolite on glycine, gamma-aminobutyric acidA, and N-methyl-D-aspartate receptors expressed in Xenopus oocytes". Anesthesia and Analgesia. 100 (5): 1400–5, table of contents. doi:10.1213/01.ANE.0000150961.24747.98. PMID 15845694.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ogata J, Minami K, Uezono Y; et al. (2004). "The inhibitory effects of tramadol on 5-hydroxytryptamine type 2C receptors expressed in Xenopus oocytes". Anesthesia and Analgesia. 98 (5): 1401–6, table of contents. PMID 15105221.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Shiraishi M, Minami K, Uezono Y, Yanagihara N, Shigematsu A, Shibuya I (2002). "Inhibitory effects of tramadol on nicotinic acetylcholine receptors in adrenal chromaffin cells and in Xenopus oocytes expressing alpha 7 receptors". British Journal of Pharmacology. 136 (2): 207–16. doi:10.1038/sj.bjp.0704703. PMC 1573343. PMID 12010769.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Shiraishi M, Minami K, Uezono Y, Yanagihara N, Shigematsu A (2001). "Inhibition by tramadol of muscarinic receptor-induced responses in cultured adrenal medullary cells and in Xenopus laevis oocytes expressing cloned M1 receptors". The Journal of Pharmacology and Experimental Therapeutics. 299 (1): 255–60. PMID 11561087.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Shiga Y, Minami K, Shiraishi M; et al. (2002). "The inhibitory effects of tramadol on muscarinic receptor-induced responses in Xenopus oocytes expressing cloned M(3) receptors". Anesthesia and Analgesia. 95 (5): 1269–73, table of contents. PMID 12401609.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Driessen B, Reimann W (1992). "Interaction of the central analgesic, tramadol, with the uptake and release of 5-hydroxytryptamine in the rat brain in vitro". British Journal of Pharmacology. 105 (1): 147–51. PMC 1908625. PMID 1596676.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bamigbade TA, Davidson C, Langford RM, Stamford JA (1997). "Actions of tramadol, its enantiomers and principal metabolite, O-desmethyltramadol, on serotonin (5-HT) efflux and uptake in the rat dorsal raphe nucleus". British Journal of Anaesthesia. 79 (3): 352–6. PMID 9389855.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Reimann W, Schneider F (1998). "Induction of 5-hydroxytryptamine release by tramadol, fenfluramine and reserpine". European Journal of Pharmacology. 349 (2–3): 199–203. PMID 9671098.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Gobbi M, Mennini T (1999). "Release studies with rat brain cortical synaptosomes indicate that tramadol is a 5-hydroxytryptamine uptake blocker and not a 5-hydroxytryptamine releaser". European Journal of Pharmacology. 370 (1): 23–6. PMID 10323276.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Ogata, J; Minami; Uezono; Okamoto; Shiraishi; Shigematsu; Ueta (2004). "The inhibitory effects of tramadol on 5-hydroxytryptamine type 2C receptors expressed in Xenopus oocytes". Anesthesia and analgesia. 98 (5): 1401–6, table of contents. PMID 15105221.
- ^ Koji Hara, M.D.; Takeyoshi Sata, M.D. (2005), "The Effects of Tramadol and Its Metabolite on Glycine, -Aminobutyric AcidA, and N-Methyl-d-Aspartate Receptors Expressed in Xenopus Oocytes", Anesthesia & Analgesia (100): 1400–1405, doi:10.1213/01.ANE.0000150961.24747.98, retrieved 2010-01-02
- ^ Barsotti, CE; Mycyk; Reyes (2003). "Withdrawal syndrome from tramadol hydrochloride". The American journal of emergency medicine. 21 (1): 87–8. doi:10.1053/ajem.2003.50039. PMID 12563592.
- ^ Choong, K; Ghiculescu (2008). "Iatrogenic neuropsychiatric syndromes". Australian family physician. 37 (8): 627–9. PMID 18704211.
- ^ Ripamonti, C; Fagnoni; De Conno (2004). "Withdrawal syndrome after delayed tramadol intake". The American journal of psychiatry. 161 (12): 2326–7. doi:10.1176/appi.ajp.161.12.2326. PMID 15569913.
- ^ "Withdrawal syndrome and dependence: tramadol too". Prescrire international. 12 (65): 99–100. 2003. PMID 12825576.
- ^ Senay, EC; Adams; Geller; Inciardi; Muñoz; Schnoll; Woody; Cicero (2003). "Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur". Drug and alcohol dependence. 69 (3): 233–41. doi:10.1016/S0376-8716(02)00321-6. PMID 12633909.
- ^ Rossi, 2004[verification needed]
- ^ a b "Tramadol Listed as Schedule IV Substance in Kentucky" (PDF) (Press release). Kentucky Board of Pharmacy. 8 December 2008. Retrieved 8 February 2009.
- ^ Jovanović-Cupić, V; Martinović; Nesić (2006). "Seizures associated with intoxication and abuse of tramadol". Clinical toxicology. 44 (2): 143–6. doi:10.1080/1556365050014418. PMID 16615669.
- ^ King, Steven A. (1 June 2007). "NSAIDs and Cardiovascular Disease". Psychiatric Times. 24 (7). Retrieved 1 August 2007.
- ^ Rodriguez, RF; Bravo; Castro; Montoya; Castillo; Castillo; Daza; Restrepo; Rodriguez (2007). "Incidence of weak opioids adverse events in the management of cancer pain: a double-blind comparative trial". Journal of palliative medicine. 10 (1): 56–60. doi:10.1089/jpm.2006.0117. PMID 17298254.
- ^ Adams, EH; Breiner, S; Cicero, TJ; Geller, A; Inciardi, JA; Schnoll, SH; Senay, EC; Woody, GE (2006). "A comparison of the abuse liability of tramadol, NSAIDs, and hydrocodone in patients with chronic pain" (PDF). Journal of pain and symptom management. 31 (5): 465–76. doi:10.1016/j.jpainsymman.2005.10.006. PMID 16716877.
- ^ Vorsanger, GJ; Xiang; Gana; Pascual; Fleming (2008). "Extended-release tramadol (tramadol ER) in the treatment of chronic low back pain". Journal of opioid management. 4 (2): 87–97. PMID 18557165.
- ^ Erowid[unreliable source?]
- ^ http://www.lakemedelsverket.se/Tpl/NewsPage____7342.aspx
- ^ http://www.buytramadolhcl.net/new-legislation-aimed-at-the-military.html
- ^ a b c FDA AccessData entry for Tramadol Hydrochloride, accessed August 17, 2009
- ^ US patent 6254887, Miller, Ronald Brown, et al., "Controlled Release Tramadol", issued 3 July 2001
- ^ US patent 7074430, Miller, Ronald Brown, et al., "Controlled Release Tramadol Tramadol Formulation", issued 11 July 2006
- ^ "Par Pharmaceutical Wins on Invalidity in Ultram(R) ER Litigation" (Press release). Par Pharmaceutical. 17 August 2009. Retrieved 29 September 2009.
External links
- Grünenthal website
- Medline Plus - Patient Information Medline Plus (A Service Of The U.S. National Library of Medicine)
- Package Insert For Tramadol Prescribing Information
