Tin(IV) bromide
 | |
 | |
| Names | |
|---|---|
| IUPAC name
tetrabromostannate
| |
| Other names
tin tetrabromide, stannic bromide, bromostannic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.258 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SnBr4 | |
| Molar mass | 438.33 g/mol |
| Appearance | colourless [1] |
| Density | 3.340 g/cm3 (at 35 °C)[1] |
| Melting point | 31 °C (88 °F; 304 K)[1] |
| Boiling point | 205 °C (401 °F; 478 K)[1] |
| soluble | |
| −149.0·10−6 cm3/mol | |
| Related compounds | |
Other anions
|
Tin(IV) fluoride Tin(IV) chloride Tin(IV) iodide |
Other cations
|
Carbon tetrabromide Silicon tetrabromide Germanium tetrabromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tin(IV) bromide is the chemical compound SnBr4. It is a colourless low melting solid.[1]
Structure
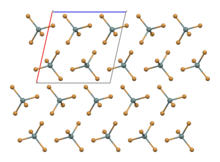
[edit]SnBr4 crystallises in a monoclinic crystal system with molecular SnBr4 units that have distorted tetrahedral geometry.[2] The mean Sn-Br bond length is 242.3 pm.[3]
Preparation
[edit]SnBr4 can be prepared by reaction of the elements at standard temperature and pressure (STP):[4][page needed]
- Sn + 2Br
2 → SnBr
4
Reactions
[edit]In aqueous solution SnBr4 dissolves to give a series of octahedral (six-ligated) bromo-aquo complexes. These include SnBr4(H2O)2 and cis- and trans-[SnBr2(H2O)4]2+.[5]
SnBr4 forms 1:1 and 1:2 complexes with ligands. With trimethylphosphine both SnBr4·P(CH3)3 and SnBr4·2P(CH3)3.[6]
Tin(IV) bromide undergoes redistribution with tin(IV) chloride as assessed by 119Sn NMR and Raman spectroscopy. Equilibrium is achieved in seconds at room temperature. By contrast, halide exchange for related germanium and especially silicon halides is slower.[7]
References
[edit]- ^ a b c d e Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Brand, P.; Sackmann, H. (1963). "Die Kristallstruktur von SnBr4" [The Crystal Structure of SnBr4]. Acta Crystallographica (in German). 16 (6): 446–451. Bibcode:1963AcCry..16..446B. doi:10.1107/S0365110X63001250.
- ^ Reuter, H.; Pawlak, R. (2001). "Zinnhalogenverbindungen. II. Die Molekül- und Kristallstrukturen von Zinn(IV)-bromid und -iodid" [Tin halogen compounds. II. The Molecular and Crystal Structures of Tin(IV) Bromide and Tin(IV) Iodide]. Zeitschrift für Kristallographie – Crystalline Materials (in German). 216 (1–2001): 34–38. Bibcode:2001ZK....216...34R. doi:10.1524/zkri.216.1.34.18992. S2CID 94609783.
- ^ Wiberg, Egon; Wiberg, Nils; Holleman, Arnold Frederick (2001). Inorganic Chemistry. Academic Press, Elsevier. ISBN 978-0-12-352651-9. OCLC 1024925228.
- ^ Taylor, M. J.; Coddington, J. M. (1992). "The constitution of aqueous tin(IV) chloride and bromide solutions and solvent extracts studied by 119Sn NMR and vibrational spectroscopy". Polyhedron. 11 (12): 1531–1544. doi:10.1016/S0277-5387(00)83148-4.
- ^ Frieson, D. K.; Ozin, G. A. (1973). "Preparation, Infrared and Raman Spectra, and Stereochemistries of Pentacoordinate Trimethylphosphine Complexes, MX4•P(CH3)3 and MX4•P(CD3)3 where M = Ge or Sn and X = Cl or Br". Canadian Journal of Chemistry. 51 (16): 2697–2709. doi:10.1139/v73-406.
- ^ Lockhart, J. C. (1965). "Redistribution and Exchange Reactions in Groups IIB-VIIB". Chemical Reviews. 65: 131–151. doi:10.1021/cr60233a004.
