Talk:Lead/Archieved
Chloride complexes
[edit]
Lead(II) forms a series of complexes with chloride, the formation of which alters the corrosion chemistry of the lead. This will tend to limit the solubility of lead in saline media.
| Pb2+ + Cl− → PbCl+ | K1 = 12.59 |
| PbCl+ + Cl− → PbCl2 | K2 = 14.45 |
| PbCl2 + Cl− → PbCl3− | K3 = 3.98 ×10−1 |
| PbCl3− + Cl− → PbCl42− | K4 = 8.92 × 10−2 |
Phase diagrams of solubilities
[edit]Lead(II) sulfate is poorly soluble, as can be seen in the following diagram showing addition of SO42− to a solution containing 0.1 M of Pb2+. The pH of the solution is 4.5, as above that, Pb2+ concentration can never reach 0.1 M due to the formation of Pb(OH)2. Observe that Pb2+ solubility drops 10,000 fold as SO42− reaches 0.1 M.

|

|
| Plot showing aqueous concentration of dissolved Pb2+ as a function of SO42− [1] | Diagram for lead in sulfate media[1] |
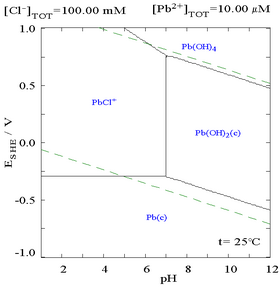
The addition of chloride can lower the solubility of lead, though in chloride-rich media (such as aqua regia) the lead can become soluble again as anionic chloro-complexes.

|
|
| Diagram showing the solubility of lead in chloride media. The lead concentrations are plotted as a function of the total chloride present.[1] | Pourbaix diagram for lead in chloride (0.1 M) media[1] |
Health effects
[edit]Lead is a poisonous metal that can damage nervous connections (especially in young children) and cause blood and brain disorders. Lead poisoning typically results from ingestion of food or water contaminated with lead; but may also occur after accidental ingestion of contaminated soil, dust, or lead based paint.[3] Long-term exposure to lead or its salts (especially soluble salts or the strong oxidant PbO2) can cause nephropathy, and colic-like abdominal pains. The effects of lead are the same whether it enters the body through breathing or swallowing. Lead can affect almost every organ and system in the body. The main target for lead toxicity is the nervous system, both in adults and children. Long-term exposure of adults can result in decreased performance in some tests that measure functions of the nervous system. It may also cause weakness in fingers, wrists, or ankles. Lead exposure also causes small increases in blood pressure, particularly in middle-aged and older people and can cause anemia. Exposure to high lead levels can severely damage the brain and kidneys in adults or children and ultimately cause death. In pregnant women, high levels of exposure to lead may cause miscarriage. Chronic, high-level exposure have shown to reduce fertility in males.[4] The antidote/treatment for lead poisoning consists of dimercaprol and succimer.
| NFPA 704 | ||||
|---|---|---|---|---|
| ||||
| Fire diamond for lead granules | ||||
The concern about lead's role in cognitive deficits in children has brought about widespread reduction in its use (lead exposure has been linked to learning disabilities).[5] Most cases of adult elevated blood lead levels are workplace-related.[6] High blood levels are associated with delayed puberty in girls.[7] Lead has been shown many times to permanently reduce the cognitive capacity of children at extremely low levels of exposure.[8]
During the 20th century, the use of lead in paint pigments was sharply reduced because of the danger of lead poisoning, especially to children.[9][10] By the mid-1980s, a significant shift in lead end-use patterns had taken place. Much of this shift was a result of the U.S. lead consumers' compliance with environmental regulations that significantly reduced or eliminated the use of lead in non-battery products, including gasoline, paints, solders, and water systems. Lead use is being further curtailed by the European Union's RoHS directive. Lead may still be found in harmful quantities in stoneware, vinyl (such as that used for tubing and the insulation of electrical cords), and brass manufactured in China. Between 2006 and 2007 many children's toys made in China were recalled, primarily due to lead in paint used to color the product.
Older houses may still contain substantial amounts of lead paint. White lead paint has been withdrawn from sale in industrialized countries, but the yellow lead chromate is still in use; for example, Holland Colours Holcolan Yellow. Old paint should not be stripped by sanding, as this produces inhalable dust.
Lead salts used in pottery glazes have on occasion caused poisoning, when acidic drinks, such as fruit juices, have leached lead ions out of the glaze.[11] It has been suggested that what was known as "Devon colic" arose from the use of lead-lined presses to extract apple juice in the manufacture of cider. Lead is considered to be particularly harmful for women's ability to reproduce. Lead(II) acetate (also known as sugar of lead) was used by the Roman Empire as a sweetener for wine, and some consider this to be the cause of the dementia that affected many of the Roman Emperors.[12]
Lead as a soil contaminant is a widespread issue, since lead is present in natural deposits and may also enter soil through (leaded) gasoline leaks from underground storage tanks or through a wastestream of lead paint or lead grindings from certain industrial operations.
Lead can also be found listed as a criteria pollutant in the United States Clean Air Act section 108. Lead that is emitted into the atmosphere can be inhaled, or it can be ingested after it settles out of the air. It is rapidly absorbed into the bloodstream and is believed to have adverse effects on the central nervous system, the cardiovascular system, kidneys, and the immune system.[13]
Biochemistry of lead poisoning
[edit]In the human body, lead inhibits porphobilinogen synthase and ferrochelatase, preventing both porphobilinogen formation and the incorporation of iron into protoporphyrin IX, the final step in heme synthesis. This causes ineffective heme synthesis and subsequent microcytic anemia.[14] At lower levels, it acts as a calcium analog, interfering with ion channels during nerve conduction. This is one of the mechanisms by which it interferes with cognition. Acute lead poisoning is treated using disodium calcium edetate: the calcium chelate of the disodium salt of ethylene-diamine-tetracetic acid (EDTA). This chelating agent has a greater affinity for lead than for calcium and so the lead chelate is formed by exchange. This is then excreted in the urine leaving behind harmless calcium.[15]
Leaching of lead from metal surfaces
[edit]The Pourbaix diagram below shows that lead is more likely to corrode in a citrate medium than it is in a non-complexing medium. The central part of the diagram shows that lead metal oxidizes more easily in the citrate medium than in normal water.

|

|
| The Pourbaix diagram for lead in a non-complexing aqueous medium (e.g., perchloric acid/sodium hydroxide)[1] | The Pourbaix diagram for lead in citric acid/citrate[1] |
In a Pourbaix diagram, the acidity is plotted on the x axis using the pH scale, while how oxidizing/reducing nature of the system is plotted on the y axis in terms of volts relative to the standard hydrogen electrode. The diagram shows the form of the element which is most chemically stable at each point, it only comments on thermodynamics and it says nothing about the rate of change (kinetics).
Exposure pathways
[edit]Exposure to lead and lead chemicals can occur through inhalation, ingestion and dermal contact. Most exposure occurs through ingestion or inhalation; in the U.S. the skin exposure is unlikely as leaded gasoline additives are no longer used. Lead exposure is a global issue as lead mining and lead smelting are common in many countries. Most countries have stopped using lead-containing gasoline by 2007.[16]
Lead exposure mostly occurs through ingestion. Lead paint is the major source of lead exposure for children. As lead paint deteriorates, it peels, is pulverized into dust and then enters the body through hand-to-mouth contact or through contaminated food, water or alcohol. Ingesting certain home remedy medicines may also expose people to lead or lead compounds.[16] Lead can be ingested through fruits and vegetables contaminated by high levels of lead in the soils they were grown in. Soil is contaminated through particulate accumulation from lead in pipes, lead paint and residual emissions from leaded gasoline that was used before the Environment Protection Agency issue the regulation around 1980.[17]
Inhalation is the second major pathway of exposure, especially for workers in lead-related occupations. Almost all inhaled lead is absorbed into the body, the rate is 20–70% for ingested lead; children absorb more than adults.[16]
Dermal exposure may be significant for a narrow category of people working with organic lead compounds, but is of little concern for general population. The rate of skin absorption is also low for inorganic lead.[16]
According to Agency for Toxic Substance and Disease Registry, a small amount of lead (1%) will store itself in bones and the rest will be excreted through urine and feces within a few weeks of exposure. Children have a harder time excreting lead. Only about 32% of lead will be excreted by a child.[18]
Occupational exposure
[edit]Lead is widely used in the production of batteries, metal products (solder and pipes), ammunition and devices to shield X-rays leading to its exposure to the people working in these industries. Use of lead in gasoline, paints and ceramic products, caulking, and pipe solder has been dramatically reduced in recent years because of health concerns. Ingestion of contaminated food and drinking water is the most common source of lead exposure in humans. Exposure can also occur via inadvertent ingestion of contaminated soil/dust or lead-based paint.
Testing
[edit]Water contamination can be tested with commercially available kits. Analysis of lead in whole blood is the most common and accurate method of assessing lead exposure. Erythrocyte protoporphyrin (EP) tests can also be used to measure lead exposure, but are not as sensitive at low blood lead levels (<0.2 mg/L). Lead in blood reflects recent exposure. Bone lead measurements are an indicator of cumulative exposure. While measurements of urinary lead levels and hair have been used to assess lead exposure, they are not reliable.
Pharmaceuticals also require laboratory testing for heavy metals such as lead. The component limit of lead (1.0 μg/g) is a test benchmark for pharmaceuticals, representing the maximum daily intake an individual should have. However, even at this low level, a prolonged intake can be hazardous to human beings.[19][20]
- ^ a b c d e f g Puigdomenech, Ignasi (2004). Hydra/Medusa Chemical Equilibrium Database and Plotting Software. KTH Royal Institute of Technology.
- ^ Ward, C. H.; Hlousek, Douglas A.; Phillips, Thomas A.; Lowe, Donald F. (2000). Remediation of Firing Range Impact Berms. CRC Press. ISBN 1566704626.
- ^ "ToxFAQs: CABS/Chemical Agent Briefing Sheet: Lead" (PDF). Agency for Toxic Substances and Disease Registry/Division of Toxicology and Environmental Medicine. 2006.
- ^ Golub, Mari S., ed. (2005). "Summary". Metals, fertility, and reproductive toxicity. Boca Raton, Fla.: Taylor and Francis. p. 153. ISBN 9780415700405.
- ^ Hu, Howard (1991). "Knowledge of diagnosis and reproductive history among survivors of childhood plumbism". American Journal of Public Health. 81 (8): 1070–1072. doi:10.2105/AJPH.81.8.1070. PMC 1405695. PMID 1854006.
- ^ "NIOSH Adult Blood Lead Epidemiology and Surveillance". United States National Institute for Occupational Safety and Health. Retrieved 2007-10-04.
- ^ Schoeters, Greet; Den Hond, Elly; Dhooge, Willem; Van Larebeke, Nik; Leijs, Marike (2008). "Endocrine Disruptors and Abnormalities of Pubertal Development". Basic & Clinical Pharmacology & Toxicology. 102 (2): 168–175. doi:10.1111/j.1742-7843.2007.00180.x. PMID 18226071.
- ^ Needleman, Herbert L.; Schell, Alan; Bellinger, David; Leviton, Alan; Allred, Elizabeth N. (1990). "The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report". New England Journal of Medicine. 322 (2): 83–88. doi:10.1056/NEJM199001113220203. PMID 2294437.
- ^ "Download: Lead paint: Cautionary note" (PDF). Queensland Government. Retrieved 7 April 2007.
- ^ "Lead Paint Information". Master Painters, Australia. Retrieved 7 April 2007.
- ^ "CPG Sec. 545.450 Pottery (Ceramics); Import and Domestic – Lead Contamination". U.S. Food and Drug Administration. Retrieved 2010-02-02.
- ^ Angier, Natalie (August 21, 2007). "The Pernicious Allure of Lead". New York Times. Retrieved 7 May 2010.
- ^ Bergeson, Lynn L. (2008). "The proposed lead NAAQS: Is consideration of cost in the clean air act's future?". Environmental Quality Management. 18: 79. doi:10.1002/tqem.20197.
- ^ Cohen, Alan R.; Trotzky, Margret S.; Pincus, Diane (1981). "Reassessment of the Microcytic Anemia of Lead Poisoning". Pediatrics. 67 (6): 904–906. PMID 7232054.
- ^ Laurence, D. R. (1966). Clinical Pharmacology(Third Edition).
- ^ a b c d "Case Studies in Environmental Medicine Lead (Pb) Toxicity: How are People Exposed to Lead?". Agency for Toxic Substances and Disease Registry.
- ^ "Information for the Community Lead Toxicity". Agency for Toxic Substances and Disease Registry.
- ^ "Toxic Substances Portal – Lead". Agency for Toxic Substance and Disease Registry.
- ^ Heavy Metals Testing By Usp. Caspharma.com. Retrieved on 2012-01-23.
- ^ pharmaceutical – Britannica Online Encyclopedia. Britannica.com. Retrieved on 2012-01-23.

