Talk:Ernest Rutherford/Archive 2
| This is an archive of past discussions about Ernest Rutherford. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 1 | Archive 2 | Archive 3 | Archive 4 |
Proposed New Section "Rutherford Transmutation Myth"
< proposed section>
A long-standing myth existed, at least as early as 1948, [1] running at least to 2017, that Rutherford was the first scientist to observe and report an artificial transmutation of a stable element into another element; nitrogen into oxygen. It was thought by many people to be one of Rutherford's greatest accomplishments. [2, 3] The New Zealand government even commemorated a stamp in honor of its belief that that the nitrogen-to-oxygen discovery belonged to Rutherford. [4]
Beginning in 2017, many scientific institutions corrected their versions of this history to indicate that the discovery credit belonged to Patrick Blackett. These institutions included the U.S. Department of Energy Office of History and Heritage Resources [5], the American Institute of Physics Center for History of Physics [6], Imperial College London, Faculty of Natural Sciences [7], and the University of Cambridge, Department of Physics. [8]
The earliest known instance of the incorrect attribution of the discovery is an article written by Rutherford in the McGill News, September 1932. The article was reprinted in the Journal of the Royal Astronomical Society of Canada in 1933.[9] In this article, Rutherford, writing about himself in the third person, claimed the discovery as his own "Rutherford, in 1919, bombarded nitrogen gas with swift alpha particles from radioactive substances and found that high-speed hydrogen nuclei, or free protons as we now term them, were liberated. … This was the first time that definite evidence had been obtained that an atom could be transmuted by artificial methods…"
Rutherford made the same claim the following year. On October 11, 1933, he gave a lecture, broadcast by the British Broadcasting Corporation, called “The Transmutation of the Atom.” A transcript of the lecture was published in The Scientific Monthly in January 1934. [10] Rutherford wrote "I made in 1919 some experiments to test whether any evidence of transformation could be obtained when alpha particles were used to bombard matter. … This was the first time that definite evidence was obtained that an atom could be transformed by artificial methods."
</proposed section>
- 1. https://www.nature.com/articles/162841b0
- 2. https://physicsworld.com/a/what-was-rutherfords-greatest/
- 3. https://royalsocietypublishing.org/doi/abs/10.1098/rspa.1964.0245
- 4. https://cerncourier.com/inside-story-the-genius-of-rutherford-revisited/
- 5. https://www.osti.gov/opennet/manhattan-project-history/Events/1890s-1939/exploring.htm
- 6. http://history.aip.org/history/exhibits/rutherford/sections/atop-physics-wave.html
- 7. http://www.imperial.ac.uk/physics/about/department-history/ (Click on Nobel Prize Winners)
- 8. http://www.cambridgephysics.org/cockcroftwalton/cockcroftwalton2_1.htm
- 9. http://adsabs.harvard.edu/full/1933JRASC..27..155R
- 10. https://www.jstor.org/stable/15525?seq=1#page_scan_tab_contents
If someone knows how to format these references correctly I would appreciate your help.
StevenBKrivit (talk) 18:40, 8 June 2019 (UTC)
- Hi StevenBKrivit. This is very interesting material. This is original research on your part but you have included a reliable secondary source as a reference, the American Institute of Physics online exhibit material that includes your findings. Give me a bit and I will format your references for you. StarryGrandma (talk) 22:52, 8 June 2019 (UTC)
- Thank you StarryGrandma If you find any part of it missing a citation to a reliable, published source, please let me know. Most likely, I can find a source. Or if you think there should be secondary or teriatary sources, please let me know that as well.
- StevenBKrivit (talk) 00:08, 9 June 2019 (UTC)
- Did ER in 1933-34 specifically claim to have identified O as a product of the alpha-N reaction in 1919? I have not read all the sources given, but it seems to me that the observation of H (or H+) was sufficient to conclude in 1919 that some transmutation (or "transformation") had taken place. Then Blackett (working in Rutherford's lab) investigated further and eventually characterized the reaction more completely. Do ER's claims in 1933-34 go beyond this?
- I agree there is a myth in the sense that elementary chemistry textbooks often oversimplify the history and just say that ER in 1919 observed He + N → H + O, but I don't think this later oversimplification should be blamed on Rutherford himself. Since he did identify H as a product, I think that the words "incorrect attribution of the discovery" are exaggerated. Dirac66 (talk) 11:16, 9 June 2019 (UTC)
- Not just elementary chemistry textbooks. See Nuclear reaction#History. Rutherford said in 1932, "This was the first time that definite evidence had been obtained that an atom could be transmuted by artificial methods…" It wasn't. The definite evidence was produced by Blackett in 1924, published in 1925, and did not confirm Rutherford's hypothesis that a collision knocked a proton from the nitrogen nucleus.
- Rutherford tended to rewrite history a bit. His 1919 paper gave the rationale for the experiment as: "These "natural" scintillations are believed to be due mainly to swift H atoms from the radioactive source, but it is difficult to decide whether thay are expelled from the radioactive source itself or are due to the action of α particles on occluded hydrogen." He unexpectedly discovered the effect on nitrogen. But in his 1935 radio interview he says: "It became clear that, to effect a veritable transformation of an atom, it was necessary to change the charge or mass of a nucleus or both together. Now, the minute nuclei of atoms are held together by powerful forces, and to effect their disintegration, it seemed likely that a very concentrated source of energy must be applied to the individual atom. The bombardment of the nuclei by the energetic alpha particles from radium appeared to be the most promising method for such a purpose. Acting on these views, I found in 1919 that the nitrogen nuclei could be transformed by bombarding them with swift alpha particles; hydrogen nuclei, or protons, as we now term them, being ejected with high speed as a result of the transformation."
- Rutherford was a towering figure in physics in the 1930s, which probably contributed just as much to people assuming he was responsible for the whole discovery. StarryGrandma (talk) 23:57, 9 June 2019 (UTC)
- Hello Dirac66.
- Hello Dirac66.
- You asked "Did ER in 1933-34 specifically claim to have identified O as a product of the alpha-N reaction in 1919?"
- You asked "Did ER in 1933-34 specifically claim to have identified O as a product of the alpha-N reaction in 1919?"
- Rutherford did not, in any of the three references I know of, specifically claim to have identified oxygen as a product of the alpha bombardment of nitrogen in the experiments he reported in 1919. Instead, he claimed, at least three times, from 1932-1935, that he transmuted or transformed nitrogen — and omitted to state exactly what he had transmuted nitrogen into.
- The third instance I found is in a video recording made by the Institution of Electrical Engineers, December 1935. Rutherford said "I found in 1919 that the nitrogen nuclei could be transformed by bombarding them with swift alpha particles; hydrogen nuclei, or protons, as we now term them, being ejected with high speed as a result of the transformation." https://www.youtube.com/watch?time_continue=1&v=zBHD8ksx_Sg
- The third instance I found is in a video recording made by the Institution of Electrical Engineers, December 1935. Rutherford said "I found in 1919 that the nitrogen nuclei could be transformed by bombarding them with swift alpha particles; hydrogen nuclei, or protons, as we now term them, being ejected with high speed as a result of the transformation." https://www.youtube.com/watch?time_continue=1&v=zBHD8ksx_Sg
- You wrote "it seems to me that the observation of H (or H+) was sufficient to conclude in 1919 that some transmutation (or "transformation") had taken place."
- But he didn't. Not in 1919. Here's what he concluded at the time:
- "From the results so far obtained, it is difficult to avoid the conclusion that the long-range atoms arising from the collision of alpha particles with nitrogen are not nitrogen atoms but probably charged atoms of hydrogen or atoms of mass 2. If this be the case, we must conclude that the nitrogen atom is disintegrated under the intense forces developed in a close collision with a swift alpha particle, and that the hydrogen atom which is liberated formed a constituent part of the nitrogen nucleus." (Collisions IV, p. 586, ¶ 1)
- "Considering the enormous intensity of the forces brought into play, it is not so much a matter of surprise that the nitrogen atom should suffer disintegration as that the alpha particle itself escapes disruption into its constituents." (Collisions IV, p. 587, ¶ 1)
- "Considering the enormous intensity of the forces brought into play, it is not so much a matter of surprise that the nitrogen atom should suffer disintegration as that the alpha particle itself escapes disruption into its constituents." (Collisions IV, p. 587, ¶ 1)
- You wrote, "Then Blackett (working in Rutherford's lab) investigated further and eventually characterized the reaction more completely."
- See if these facts help:
- The conclusion of Rutherford's four Collisions papers reported his experimental evidence that showed that hydrogen atoms were indeed a reaction product of the alpha bombardment of nitrogen rather than a direct emission of the radioactive source. This is the primary objective he sought to resolve from Marsden; Rutherford had succeeded in his goal.
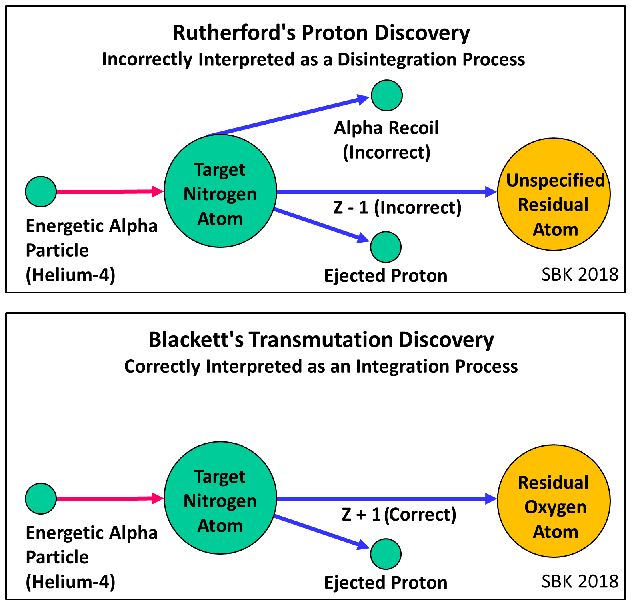
- On the other hand, nothing in Rutherford's Collisions papers concerned a) the identity of the residual nucleus after alpha bombardment, b) any discussion of transmutation of elements or the pursuit of evidence of elemental transmutation, c) the identification of any new element from transmutation or d) the correct interpretation of the process that resulted in the emission of the proton and the transmutation of nitrogen to oxygen.
- After completing his 1919 work, Rutherford knew that the identity of the residual nucleus was an unanswered question. He assigned Blackett to resolve that question. For the next four years, Blackett performed the experiments, obtained the data, correctly analyzed the primary process as one of integration rather than disintegration, corrected Rutherford's wrong assumption that the alpha particle remained intact, and published his data and his conclusion in 1925.
- After completing his 1919 work, Rutherford knew that the identity of the residual nucleus was an unanswered question. He assigned Blackett to resolve that question. For the next four years, Blackett performed the experiments, obtained the data, correctly analyzed the primary process as one of integration rather than disintegration, corrected Rutherford's wrong assumption that the alpha particle remained intact, and published his data and his conclusion in 1925.
- You wrote "I agree there is a myth in the sense that elementary chemistry textbooks often oversimplify the history and just say that ER in 1919 observed He + N → H + O"...
- The myth and the "oversimplification" existed in more than "elementary chemistry textbooks."
- See if these facts help:
- The myth and the "oversimplification" existed in more than "elementary chemistry textbooks."
- "The first artificial nuclear transmutation to be recognized and described as such was reported by Rutherford, 1919. ... The results of this experimental work correctly interpreted by Rutherford as a real nuclear disintegration of nitrogen nuclei into oxygen atoms and protons, resulting from the bombardment of nitrogen atoms by the alpha particles." (Sharma, B.K. (2001) Nuclear and Radiation Chemistry, Krishna Prakashan Media)
- "Blackett, also at the Cavendish, used a Wilson chamber in the early 1920s to confirm Ernest Rutherford's transmutation of nitrogen into oxygen." (Westwick, Peter J. (2005) "Cherenkov Radiation," in Oxford Guide to the History of Physics and Astronomy, Heilbron, J.L., ed., Oxford University Press, ISBN-10: 0195171985, p. 1)
- "In 1919, Rutherford succeeded [with] ... his wartime discovery that alpha particles from radioactive substances can transmute nitrogen into oxygen." (Heilbron, J.L., ed. (June 3, 2005) Oxford Guide to the History of Physics and Astronomy, Oxford University Press)
- "Blackett later proved, with the cloud chamber, that the nitrogen in this process was actually transformed into an oxygen isotope, so that Rutherford was the first to deliberately transmute one element into another." (Nobelprize.org, 2014)
- "By 1918 Rutherford, in Manchester, had proved that a nitrogen atom could be disintegrated into an oxygen atom and a hydrogen atom. Although he hadn't produced gold, Rutherford had fulfilled the alchemists' dream of turning one element into another! He published his research in 1919, the year he became the Cavendish Professor." (University of Cambridge physics Web site, retrieved 3/13/2017)
- "Blackett started his research career in the 1920s with Rutherford, who had discovered the transmutation of nitrogen to oxygen." (Imperial College of London Web site retrieved March 26, 2014)
- "The first artificial nuclear transmutation to be recognized and described as such was reported by Rutherford, 1919. ... The results of this experimental work correctly interpreted by Rutherford as a real nuclear disintegration of nitrogen nuclei into oxygen atoms and protons, resulting from the bombardment of nitrogen atoms by the alpha particles." (Sharma, B.K. (2001) Nuclear and Radiation Chemistry, Krishna Prakashan Media)
- You wrote "Since [Rutherford] did identify H as a product, I think that the words 'incorrect attribution of the discovery' are exaggerated."
- I'm having trouble following your point. Are you arguing that the phrase "incorrect attribution" of the nitrogen to oxygen transmutation discovery is an exaggeration because Rutherford discovered the proton?
- I almost forgot a very important reference for the prevalence of the myth: The Wikipedia Rutherford page. It existed nearly from the beginning of this page's creation. It was introduced on Jan. 23, 2003 by an editor at 132.181.3.189, which resolves to Christchurch, Canterbury, New Zealand. https://wiki.riteme.site/w/index.php?title=Ernest_Rutherford&diff=next&oldid=610143. Pre-existing similar language appears on John Alexander Campbell's page from 2001. https://web.archive.org/web/20010730160830/https://www.rutherford.org.nz/biography.htm. The myth was on the Wikipedia Rutherford page until June 24, 2018, at which time I corrected the myth.
- StevenBKrivit (talk) 16:24, 10 June 2019 (UTC)
- Hello Starry Grandma and Steven B. Krivit. Thanks for your answers. I also found useful Steven Krivit’s article in New Energy Times [1].
- My point was that ER in 1919 would have considered the appearance of protons (hydrogen ions or “atoms”) as evidence of transmutation because the observed product hydrogen is an element not initially present. I understand that he did not at that time know that the other product was oxygen, so he would have viewed the reaction as He(α) + N → H + ? (+ ?), but this would be still nuclear transmutation (or “transformation”) since the new element H is produced. Later Blackett identified the second product as oxygen, but ER still should have credit for showing that one product (H) is an element not present in the initial state, which is the definition of transmutation.
- Starry Grandma pointed out that ER also considered the possibility that the protons (H) came from the radioactive source, but Steven Krivit’s New Energy Times article says that this hypothesis came from Marsden and was disproved by ER who showed that the proton (H) was actually a product of the reaction with N. So he did show that there was indeed a nuclear reaction, even before Blackett identified the second product as O.
- Also as pointed out by Starry Grandma and also in The New Energy Times article, ER’s alternative transmutation hypothesis was: α + element Z → α + H + element Z-1, which for a nitrogen target corresponds to He + N → He + H + C. It might be interesting to mention this hypothesis in the article in order to show that ER did not consider only ideas which have turned out to be true. If we do so, however, we should help readers by saying very clearly that Blackett’s work disproved this description.
- As for the myth I think it is all right to point out that some other people erroneously state or imply that ER identified the reaction He + N → H + O. But I don’t think ER himself should be blamed for the fact that other people did not read his articles or his later comments carefully enough. If the myth dates from 1948 or 11 years after ER’s death, he did not have a chance to correct the errors. Dirac66 (talk) 22:51, 11 June 2019 (UTC)
- Hello Dirac66. Your thesis in your first paragraph requires that by 1932, Rutherford did not realize the difference between a hydrogen atom and a proton. Would you please respond to this before I respond to your other comments? Thank you.
- StevenBKrivit (talk) 23:38, 11 June 2019 (UTC)
- I don't see why my argument requires that assumption. I think the proposer of the atomic nucleus knew the difference between H and H+ from about 1908, and by 1917-19 he had renamed H+ the "proton". However when discussing the α + N reaction, he still referred (at least once) to the proton as a "hydrogen atom". I would guess this was because he realized that charge states don't matter when discussing nuclear reactions whose energies are so much greater than those of chemical reactions. The free proton eventually picks up an electron from somewhere and becomes a hydrogen atom. Even today, nuclear reactions are usually written without specifying the charge states of the reactant and product atoms. Dirac66 (talk) 01:16, 12 June 2019 (UTC)
- Hello Dirac66. Your thesis in your first paragraph requires that by 1932, Rutherford did not realize the difference between a hydrogen atom and a proton. Would you please respond to this before I respond to your other comments? Thank you.
- Let's cut to the chase please. Yes or no answers would be greatly appreciated:
- 1. Are you asserting that Rutherford transmuted nitrogen to hydrogen?
- 2. Are you asserting that by 1932 Rutherford did not know the difference between the two?
- StevenBKrivit (talk) 01:26, 12 June 2019 (UTC)
- Another point: You write "ER still should have credit for showing that one product (H) is an element not present in the initial state, which is the definition of transmutation." The fundamental discovery he is known for in these 1919 papers was the discovery of the proton, which was abundantly present in the nitrogen target.
- StevenBKrivit (talk) 01:34, 12 June 2019 (UTC)
- Moving on. Rutherford knew in 1919 that "the hydrogen atom which is liberated formed a constituent part of the nitrogen nucleus." Everyone knows that. (Collisions IV, p. 586, ¶ 1)
- I believe Dirac66, that your remaining objection is that you don't think this Wikipedia article should attribute the source of this myth to Rutherford. You propose to omit this part of the history. Instead, you propose that we should inform Wikipedia readers that the myth developed because some person or people between 1919 and 1948 did not read Rutherford's "articles or his later comments carefully enough" and that they were responsible for creating the myth. You are partially correct.
- In my research of news archives from June 1919 until December 1919, I found absolutely nothing about Rutherford and transmutation. Then, in December 1919, I found an article in the French newspaper Le Matin and had it translated to English. The author thought that Rutherford had transmuted elements; changing nitrogen into hydrogen. Rutherford, of course, knew in 1919 that, in his own words, "the hydrogen atom which is liberated formed a constituent part of the nitrogen nucleus," rather than it being an element that was not present before the experiment. He named this constituent particle "the proton" a year later. Your assertion that he transmuted, or believed he transmuted the element nitrogen into the element hydrogen is contradicted by these very simple facts.
- Le Matin missed the distinction between the element hydrogen and the soon-to-be-named subatomic particle, the proton. Le Matin ran this story six months after Rutherford published his Collisions papers and claimed that Rutherford had discovered the transmutation of elements. The Le Matin story was picked up by the Associated Press and it subsequently appeared all over the United States and perhaps abroad. A few more news stories about this dribbled out in early 1920. And then, nothing, for years. Even when Blackett's paper published in 1925, I could find no new stories about transmutation associated with him or with Rutherford. Not until we get to 1932.
- But all of that is irrelevant to your proposal. Why? Because it still leaves us with the fact that in 1932-1935, Rutherford claimed that his own experiment in 1919 "was the first time that definite evidence had been obtained that an atom could be transmuted by artificial methods…"
- So, for completeness, we could add to the proposed section: "As a result of a misunderstanding of the author of a news story in Le Matin, many news stories in winter 1919-1920 claimed Rutherford had transmuted elements."
- Your comments please.
- StevenBKrivit (talk) 04:28, 12 June 2019 (UTC)
Transmutation should be described by complete equation
I will try to answer the questions in the last two edits:
1. Are you asserting that Rutherford transmuted nitrogen to hydrogen?
Transmutation like all (nuclear or chemical) reactions is best described by a complete balanced equation, in this case 14N + 4He → 17O + 1H. The most accurate description is that N and He both react (or “are transmuted”) to two products, O and H. It is customary to ignore the light nuclei and just say that N is transmuted to O, but this can lead to confusion as in our discussion, and then it is clearer to include all four nuclei for clarity. So yes, N (and He) are transmuted to H (and O), although it is not usually expressed that way. The term transmutation for a real physical process was first proposed by Soddy to Rutherford in 1901 in order to describe the radioactive decay of thorium. By analogy with the alchemists’ fictitious Pb → Au, the word suggests an unbalanced reaction Th → Ra, but of course Soddy and Rutherford showed that it is balanced by the decay of an alpha-particle: Th → Ra + He. So strictly Th is transmuted to Ra and He, though it is not usually said that way. The emission of alpha particles from Th provides evidence that the radioactive decay occurs. Similarly, for the N + alpha reaction, the observation of hydrogen (as free protons) showed Rutherford that a nuclear reaction (transmutation) is occurring, although the other product was unidentified until Blackett’s work.
2. Are you asserting that by 1932 Rutherford did not know the difference between the two?
Sorry, what two did you mean here?
3. You write "ER still should have credit for showing that one product (H) is an element not present in the initial state, which is the definition of transmutation." The fundamental discovery he is known for in these 1919 papers was the discovery of the proton, which was abundantly present in the nitrogen target.
Yes, but ER proposed the proton in order to explain the transmutation reaction. Prior to 1917-19, the proton was known only as the free hydrogen ion H+, and not as a constituent of other nuclei such as N. My words “not present in the initial state” should have been “not known to be present in the initial state”. When ER discovered that N + He → H + ?, he proposed that N and He must contain bound hydrogen nuclei which he renamed protons. The observation of hydrogen ions (or “atoms”) from the N + He reaction (or transmutation) was his experimental evidence for the existence of protons in other nuclei. This means the early sources such as Le Matin and McGill News were correct, since they did not give ER credit for the full equation including oxygen. Dirac66 (talk) 21:25, 12 June 2019 (UTC)
- First, let's finish up the old discussion. My question 2 was not clear, my apologies. Take two: Are you asserting that by 1932 Rutherford did not know the difference between a hydrogen atom and the proton?
- StevenBKrivit (talk) 21:38, 12 June 2019 (UTC)
- No. Did I say something to suggest that? Dirac66 (talk) 23:43, 12 June 2019 (UTC)
Proposed New Section "Rutherford Transmutation Myth" (2)
Dear Dirac66,
Your statement is unsupported: "the observation of hydrogen (as free protons) showed Rutherford that a nuclear reaction (transmutation) is occurring."
This statement would be supported: "the observation of hydrogen (as free protons) showed Rutherford that a nuclear reaction is occurring."
Your statement is unsupported: "ER proposed the proton in order to explain the transmutation reaction."
This statement would be supported: "ER proposed the name proton in response to the growing recognition at the time of a new subatomic particle and to explain his developing concept of the atomic nucleus."
I'll answer my own question: Of course by 1932 Rutherford knew the difference between a hydrogen atom and the proton. The inevitable facts are that a) in 1932-35, Rutherford claimed that he transmuted elements in 1919, but in fact, he had no such evidence for doing so in 1919, b) he made no such claim in his 1919 papers, c) he only began making such claims years after Blackett definitively obtained that evidence for the first artificial transmutation, and d) Rutherford did so a few months after Cockroft and Walton revealed the dawn of high-energy physics by way of their acccellerator. Yeah, I get it. You don't want this history on the Wikipedia page. You're a physicist. You're a fan of Dirac. It doesn't look good for Rutherford. But shit happens. You've made a good attempt to argue for blocking my proposal and I salute your efforts.
At this point, I'd like to remind you and the Wikipedia community that your blocking of my proposed section based on your opinion that Rutherford should be credited with transmutation runs counter to the current independent, authoritative sources who have, within the past two years, corrected their records to remove this transmutation credit from Rutherford. These sources include the American Institute of Physics, Atomic Heritage Society (Blackett Page), Atomic Heritage Society (Rutherford Page) Britannica.com (Alpha Page), Cambridge University, Chemistry Views, Imperial College London (See N.P. Winners), Institute of Physics Digital Education, Nobel Foundation (Rutherford page), Oxford University Press, Royal Society of Chemistry, Royal Society, The Scientist, University of California Santa Barbara and the U.S. Department of Energy.
Links to all citations are on this page https://www.newenergytimes.com/v2/sr/Rutherford-Blackett/Rutherford-Blackett.shtml
It's time for me to move on. I thank you for your engagement with me on this matter.
StevenBKrivit (talk) 22:49, 12 June 2019 (UTC)
- I have reverted the recent additions. Firstly, NewEnergyTimes is not a reliable source, and this is your own writing. Secondly, what was present may well be true, however it was nonetheless a pure original synthesis of disparate sources. I'm not saying this cannot be included, but we need reliable sources do to so. It also ignores things like this cover of the Boston Globe from 1919 (on the left). Headbomb {t · c · p · b} 04:39, 20 June 2019 (UTC)
- I have reverted your revert. We have had this in discussion here since June 8. Please pay attention: New Energy Times is NOT used as any source in this addition. I can help explain the Boston Globe and other stories.
- StevenBKrivit (talk) 04:52, 20 June 2019 (UTC)
- myth stated by AIP: https://history.aip.org/history/exhibits/rutherford/sections/atop-physics-wave.html
- myth stated by DOE, see cartoon: https://www.osti.gov/opennet/manhattan-project-history/Events/1890s-1939/exploring.htm
- Thus, your basis for reverting is without merit. I am reverting your revert. Please stop groundless reversions.
- If you have further objections to this addition, please explain your objections here first and let's discuss civilly. I will be happy to respond to any arguments you provide within 24 hours. Thank you. — Preceding unsigned comment added by StevenBKrivit (talk • contribs) 05:17, 20 June 2019 (UTC)
- StevenBKrivit (talk) 05:09, 20 June 2019 (UTC)
- I've asked for further input from WT:PHYS. AIP supports that (which again traces back to your own publications, but that's at least an independent source), but there's nothing in the DOE that speaks of any myth. Headbomb {t · c · p · b} 05:14, 20 June 2019 (UTC)
- DOE: Look for the caption that says "1948 comic depicting the Rutherford transmutation MYTH." (emphasis added)
- StevenBKrivit (talk) 05:20, 20 June 2019 (UTC)
- I've asked for further input from WT:PHYS. AIP supports that (which again traces back to your own publications, but that's at least an independent source), but there's nothing in the DOE that speaks of any myth. Headbomb {t · c · p · b} 05:14, 20 June 2019 (UTC)
Detecting Hydrogen
Hello Dirac66,
Thank you for your contribution to this section. You added this sentence "In fact Rutherford did detect the hydrogen product and interpret it as evidence that some nuclear transmutation had occurred."
The consensus in the scientific community is that the primary discovery published by Rutherford in 1919 is his definitive experimental evidence for the existence of what we now know as the proton. As we know, Rutherford didn't call it a proton in 1919 because a) he had not produced the evidence for it prior to these four papers and b) he had not proposed the name for that particle yet. This is the brilliance of his 1919 discovery; he produced the evidence and therefore was on solid ground to later christen the subatomic particle with its name "proton."
However, prior to the completion of his 1919 papers, he and his colleagues did the best they could to identify this unidentified "thing" that they saw being produced from alpha-bombardment experiments. Sometimes they called it a "swift H atom" sometimes they called it a hydrogen atom.
After his 1919 papers, and by the time Rutherford suggested the name "proton" he could differentiate the single-particle proton from the three-particle hydrogen atom.
If you want, we can say that, at the time he published Collisions IV, he thought that he had liberated a hydrogen atom because this is what Rutherford said:
"the long-range atoms arising from the collision of alpha particles with nitrogen are not nitrogen atoms but probably charged atoms of hydrogen or atoms of mass 2. ...and that the hydrogen atom which is liberated formed a constituent part of the nitrogen nucleus." (Collisions IV, p. 586, ¶ 1)
Certainly, I think we can agree, by the time Rutherford named the proton in 1920, he understood the difference between a proton and a hydrogen atom. Certainly, by the time Blackett published his particle tracks, Rutherford knew that what he had described in 1919 as the ejected hydrogen atom was actually an ejected proton.
So we can say, if you really insist, that for a short period of time, Rutherford believed that he had detected the emission of a hydrogen atom. But we have no basis to say now, or even if we were alive in the late 1920s, that Rutherford had detected the emission of a hydrogen atom. The phrase as you have provided "Rutherford did detect the hydrogen product" has absolutely no support and I unequivocally suggest its removal.
If you are insistent on injecting some variation of your phrase, the only factual way I can see doing it is thus: "prior to the definitive identification and the naming of the proton, Rutherford thought that the ejected 'constituent part of the nitrogen nucleus' that he observed was a hydrogen atom."
As far as your phrase that Rutherford "interpret[ed] it as evidence that some nuclear transmutation had occurred," I must ask you to cite your source on this please or remove it.
Thank you,
StevenBKrivit (talk) 04:28, 22 June 2019 (UTC)
- Thank you for your comments. I will try to clarify my view of some questions you have mentioned:
- Hydrogen atom vs. proton: Yes, I agree that Rutherford knew the difference between a proton and a (charged) hydrogen atom, from 1919 when he proposed the proton. But after it has been ejected from another nucleus (such as nitrogen in his initial hypothesis) or formed in a nuclear reaction, the proton becomes just a fast, charged hydrogen atom H+ (not a neutral hydrogen atom H), which is what Rutherford observed. However, Rutherford realized that the (charged) “hydrogen atom” also exists inside the other nucleus and is a fundamental constituent of all nuclei. This is why he gave it the new name of proton to emphasize its importance in nuclear physics.
- Transmutation: You have questioned whether Rutherford in 1919 knew that his observations implied the occurrence of some transmutation reaction, even though he had not identified the other product(s). The point is that transmutation (or nuclear transformation) was defined by Soddy and Rutherford as a process which changes an element or elements into another element or elements. According to 19th-century chemistry, if the reactants include only nitrogen and helium atoms, the products can include only nitrogen and helium atoms. Processes which break this rule must involve some nuclear reaction. Rutherford observed the emission of hydrogen atoms which was sufficient to indicate transmutation: N + He → H + ? (+?). We have agreed that Blackett identified the second product as oxygen.
- As for sources, I would point to your own citation of Rutherford’s McGill News and BBC articles. In both he states that “This was the first time [in 1919] that definite evidence had been obtained that an atom could be transmuted/transformed by artificial methods”. I do not think this goes beyond what he actually did in 1919, since it does not mention the oxygen product.
- In reply to “If you want, we can say that, at the time he published Collisions IV, he thought that he had liberated a hydrogen atom …”, I would point out that you already included Rutherford’s words “found that high-speed hydrogen nuclei, or free protons as we now term them, were liberated”, which I have not changed.
- And as for my sentence "In fact Rutherford did detect the hydrogen product and interpret it as evidence that some nuclear transmutation had occurred, but he did not identify the other product as oxygen.", this could be improved to "In fact Rutherford did detect the charged hydrogen product and realize that it was emitted from a heavier nucleus in some nuclear transmutation reaction, although he did not identify the other product as oxygen. He suggested that the charged hydrogen atom was a component of all heavier nuclei, and renamed it the proton." Dirac66 (talk) 01:56, 23 June 2019 (UTC)
- Hi Dirac66,
- Hi Dirac66,
- This is, more or less what you said before but you've done so more clearly and thoroughly here. I appreciate your effort and your time. I see that you are attempting to find a way to support Rutherford's 1933-34 claims that he was the first to accomplish artificial transmutation.
- Your thesis, if I'm not mistaken, is that Rutherford, in 1933-34, in making his transmutation claim, was referring to, in your words, the transmutation of nitrogen into hydrogen. I'm willing to conditionally accept the matter of the hydrogen atom versus proton particle as you've described, but I still have questions for you about the history.
- 1. Did Rutherford ever explicitly say that he interpreted the hydrogen product as evidence that a nuclear transmutation had occurred? If so, what is the citation please?
- 2. Are there any credible sources besides Rutherford promoter John Campbell who has written or claimed that Rutherford had transmuted nitrogen into hydrogen? If so, what is the citation please?
- On the other hand, if we look at history, we have ample evidence that contradicts your thesis.
- A. Rutherford's Bakerian Lecture, June 3, 1920: "The expulsion of a mass 3 carrying two charges from nitrogen, probably quite independent of the release of the H atom, lowers the nuclear charge by 2 and the mass by 3. The residual atom should thus be an isotope of boron of nuclear charge 5 and mass 11. If an electron escapes as well, there remains an isotope of carbon of mass 11. The expulsion of a mass 3 from oxygen gives rise to a mass 13 of nuclear charge 6, which should be an isotope of carbon. In case of the loss of an electron as well, there remains an isotope of nitrogen of mass 13. The data at present available are quite insufficient to distinguish between these alternatives." [1]
- There is no evidence here to suggest that Rutherford thought that the H atom was THE transmutation product or even A "transmutation" product. This shows that Rutherford knew that his experiment must certainly have transmuted the nitrogen atom into something else besides the H atom. It shows that Rutherford was thinking that the residual atom, THE transmutation product, should be an element proximal to nitrogen.
- B: Rutherford's abstract for his lecture to the Royal Institution of Great Britain, On April 5, 1925: ""Since the proof that protons can be expelled from the nuclei of many light elements, the fate of the bombarding alpha particle after the disintegration has been a matter of conjecture. To throw light on this question, Blackett has recently photographed the tracks of more than 400,000 alpha particles in nitrogen. ... In these photographs, the fine track of the proton was clearly visible, and also that of the recoiling nucleus, but in no case was there any sign of a third branch due to the escaping alpha particle. He concluded that the alpha particle was captured in a collision which led to the ejection of a proton. ... These experiments suggest ... that the alpha particle is captured by the nucleus. If no electron is expelled, the resulting nucleus should have a mass 14 + 4 - 1 = 17, and a nuclear charge 7 + 2 - 1 = 8 — that is, it should be an isotope of oxygen. It thus appears that the nucleus may increase rather than diminish in mass as the result of collisions in which the proton is expelled. [2]
- Again, this shows no evidence that Rutherford was thinking that, as he now called them, protons were the transmutation product. To the contrary, he affirms Blackett's transmutation discovery that oxygen was the transmutation product and he affirms that Blackett had figured out that the primary reaction was an integration rather than a disintegration.
- I conditionally accept your assertion that the hydrogen atom and the proton particle can be argued to be the same. However, as before, I fail to see any historical support for your interpretation that "In fact Rutherford did detect the hydrogen product and interpret it as evidence that some nuclear transmutation had occurred."
- I am well aware that in late 1919 and early 1920, many newspaper reporters reported and thought that Rutherford had transmuted nitrogen into hydrogen. They are not credible sources, nor do their opinions satisfactorily represent the views of Rutherford. Rutherford's Bakerian lecture proves that by 1920 he knew his experiments had transmuted elements; he just didn't have the data to support it yet. Any time after 1925 — for example in 1933-1934 — Rutherford absolutely knew that the transmutation product of nitrogen was oxygen. To suggest otherwise is, in my humble opinion, ludicrous. But please, if you'd be so kind as to address my two questions above.
- Thank you,
- Steven
- Thank you,
- 1. Rutherford, Ernest (1920) "Nuclear Constitution of Atoms" [Bakerian Lecture], Proceedings of the Royal Society of London 97-A, 374-400; reprinted in The Collected Papers of Lord Rutherford of Nelson O.M., F.R.S. Published under the Scientific Direction of Sir James Chadwick, F.R.S., Chadwick, James, ed., 3, George Allen and Unwin, p. 14-38, (1965)
- 2. Rutherford, Ernest (1925) "Studies of Atomic Nuclei," in Proceedings of the Royal Institution Library of Science," 9, 75
- StevenBKrivit (talk) 21:51, 23 June 2019 (UTC)
- 1. Rutherford, Ernest (1920) "Nuclear Constitution of Atoms" [Bakerian Lecture], Proceedings of the Royal Society of London 97-A, 374-400; reprinted in The Collected Papers of Lord Rutherford of Nelson O.M., F.R.S. Published under the Scientific Direction of Sir James Chadwick, F.R.S., Chadwick, James, ed., 3, George Allen and Unwin, p. 14-38, (1965)
- Hi Dirac66,
- I want to mention that your thesis that a proton and a hydrogen atom are effectively the same in this context appears to be contradicted by Malcolm Longair; the Jacksonian Professor Emeritus of Natural Philosophy and former Head of the Cavendish Laboratory at the University of Cambridge. Take a look at what he said here:
- https://www.thenakedscientists.com/articles/interviews/when-rutherford-split-atom
- Hi Dirac66,
- "Rutherford carried on doing these experiments, and by 1917 he had discovered that these particles really had to be very light, fast particles coming out of the nucleus and made a suggestion that these were fast protons and that was the nuclei of hydrogen atoms."
- I would venture to say that if Longair makes the distinction between protons and hydrogen atoms, specifically with regard to the Rutherford experiment, we should too. Your thesis now appears to lack support from a physics perspective as well as a historical perspective. I will wait another 48 hours. If you can't clearly provide support for your thesis, and if you can't contradict Longair with an equally credible source, then it's time for one of us to pull your sentence.
- Thank you,
- StevenBKrivit (talk) 22:48, 23 June 2019 (UTC)
- Transmutation product: I notice that we are using two different meanings for "transmutation product", which I think has contributed to the confusion. Both meanings are found in the literature, so it is not a question of who is right and who is wrong, but I still think it is worth pointing out the difference, say for the thorium decay which was the first reaction called transmutation by Rutherford and Soddy in 1901. Then we can perhaps agree on a rewording for the N-alpha reaction.
- You are apparently using “transmutation product” to include only the product(s) with a large mass comparable to the target nucleus. For Th → Ra + He this is Ra only and not He. Of course, Rutherford and Soddy knew that He is also produced in the reaction, but instead of calling it a product of the transmutation reaction they described it as an ejected alpha-particle or some synonym thereof. I admit that this usage is more common at least in physics. For the Blackett reaction, this would include only oxygen as you have argued.
- However, I have been using "product of the transmutation reaction" in the chemist’s sense as including everything formed in the reaction: Ra and He for the decay of Th, O and H for the Blackett reaction. But I admit it doesn’t really matter if the low-mass atom is called a product or an ejected particle, as long as we understand it is there. So I will agree that we don’t need to refer to a “hydrogen product”, and I propose that we replace the sentence
- "In fact Rutherford did detect the hydrogen product and interpret it as evidence that some nuclear transmutation had occurred, but he did not identify the other product as oxygen." by
- "In fact Rutherford did detect the ejected proton and interpret it as evidence that some nuclear transmutation had occurred, but he did not identify the product as oxygen."
- If this wording is acceptable to you, go ahead and make the change. Notice that I have also replaced hydrogen atom by proton as requested. However I do object strongly to the suggestion of pulling the entire sentence. Dirac66 (talk) 01:36, 24 June 2019 (UTC)
- Hi Dirac66,
- Hi Dirac66,
- I appreciate your efforts. I think that your suggestion to replace "hydrogen product" with "ejected proton" is a huge improvement.
- I want Wikipedia to be as accurate as you do. So how do we make this phrase historically accurate: "interpret it as evidence that some nuclear transmutation."
- If we take your proposed revised sentence and modify it to this:
- "In fact, Rutherford did detect the ejected proton in 1919 and, beginning in 1932, interpreted it as evidence that some nuclear transmutation had occurred, but he never identified the product as oxygen."
- that would work for me. If you can cite an earlier reference where he personally stated it as "transmutation," then perhaps you could use that instead.
- Thank you,
- StevenBKrivit (talk) 02:41, 26 June 2019 (UTC)
- Reply: Rather than propose new sources, I have read some of yours more closely. The most relevant seems to be the one you have abbreviated as Collisions … p.586, which cites Rutherford as concluding in 1919 that the N atom is “disintegrated”. This is clearly a comparison to the radioactive disintegration of U, Th, etc. to lighter atoms, which involves the transmutation of elements as Rutherford and Soddy had already shown. The difference for Rutherford in 1919 was that the disintegration occurs “under the intense forces developed in a close collision with a swift alpha particle,” rather than naturally. But the reaction considered by Rutherford in 1919 was still disintegration and therefore a nuclear reaction or transmutation.
- Then in 1925 Blackett showed that the true product is oxygen which is heavier than nitrogen, so the word “disintegration” was no longer appropriate. But it was still a nuclear reaction or transmutation, and Rutherford could still say correctly in 1932 that he had evidence in 1919 for a transmutation, although to the wrong products. In view of this argument, the sentence we were discussing should probably contain the word “disintegration” which Rutherford actually used. I now suggest the following:
- "In fact, Rutherford did detect the ejected proton in 1919 and interpreted it as evidence for “disintegration” of the nitrogen nucleus (to lighter nuclei). However, Blackett identified the true reaction in 1925 and showed that the actual product is oxygen which is heavier than nitrogen. Beginning in 1932 therefore, Rutherford clarified his version of his discovery in 1919, stating that he had found evidence that some nuclear transmutation had occurred." This is longer but I think more accurate:
- I have also found the Le Matin story at https://gallica.bnf.fr/ark:/12148/bpt6k573081n.texteImage (oui, en français). Unfortunately the author, Charles Nordmann, does not say where he obtained his information, so this story does not really establish whether or not Rutherford used the word “transmutation”. Dirac66 (talk) 20:02, 27 June 2019 (UTC)
- Hi Dirac66,
- This looks like progress.
- In this phrase: ---- interpreted it as evidence for “disintegration” ---- I do not think the quotes belong around the word disintegration. He absolutely interpreted as evidence for a disintegration process.
- You're also missing a crucial step here: --- the true reaction in 1925 and showed that the actual product is oxygen which is heavier than nitrogen. ---
- It should be something like this: --- the true reaction in 1925 was one of integration rather than disintegration, and showed that the actual product is oxygen which is heavier than nitrogen. ---
- Rutherford himself recognized this two months later, on April 5, 1925: "It thus appears that the nucleus may increase rather than diminish in mass as the result of collisions in which the proton is expelled." [1]
- 1. Rutherford, Ernest (1925) "Studies of Atomic Nuclei," in Proceedings of the Royal Institution Library of Science," 9, 75
- Perhaps these drawings will help.
- Here's what I wrote about the Le Matin story in Lost History (page 278-9):
- The article said nothing about any recent news that might have triggered the story. My search revealed no related news at the time or for the previous five months. After I obtained a translation of the article, I learned that the underlying story in Le Matin was the same as that reported in The Independent. They were both about Rutherford's shattering of a nitrogen nucleus and the emission of a proton.
- Le Matin took a different approach from The Independent. Whereas The Independent had simply and accurately reported the nature of Rutherford's claim, Le Matin spun the story as a modern-day discovery of alchemy and transmutation. The second sentence in the Le Matin story presented Nordmann's news angle. "The transmutation of chemical elements," Nordmann wrote, "that old dream that preyed on the mystic minds of alchemists during the Middle Ages, has just been accomplished for the first time. The media, those modern, telegraphic trumpeters of goodwill, have neglected to spread the news."
- As far as I could tell, there was no triggering news event. Nordmann thought this was big news, and he blamed the lack of news coverage of Rutherford's discovery on his colleagues' "neglect." Yet in his article, too, there was nothing about transmuting nitrogen to oxygen.
- Nevertheless, the Le Matin story had a significant impact. Follow-up stories — all based on the Le Matin story — ran the next day in the U.S. from coast to coast. However, the Le Matin story had no news to report: no recent event, no science conference, no interviews, not even any direct quotes from sources.
Hello Steven
1. OK for removing quotes from the word "disintegration" in the article. (I place quotes here to indicate which is "the word" being discussed, but I will not place them in my next suggested version below.)
2. The problems with the word "integration" are a) Rutherford did not use it and b) it is not used in nuclear physics generally. Note that Wikipedia has a dab page Integration with about 30 other meanings of the word, all irrelevant to this article.
Instead I suggest using Rutherford's own 1925 words which you have now cited "increase rather than diminish in mass". The passage would now read:
However, Blackett identified the true reaction in 1925 and showed that the actual product is oxygen. Rutherford therefore recognized "that the nucleus may increase rather than diminish in mass as the result of collisions in which the proton is expelled." [1]
3. Thanks for further information re the Le Matin article. We could cite it to show that by 1919, the popular press was starting to print sensationalized descriptions of nuclear reactions as "transmutations". Dirac66 (talk) 15:10, 28 June 2019 (UTC)
- Hello Dirac66 who someday I may have the pleasure of knowing by your RL ID ;) -
- I think we have arrived at consensus. If I have tracked this correctly, your proposal above replaces this sentence?
- -----In fact Rutherford did detect the hydrogen product and interpret it as evidence that some nuclear transmutation had occurred, but he did not identify the other product as oxygen.-----
- If so, please do make the change.
- As far as the popular press and transmutations, no, that wouldn't be right. Most people younger than 80 who are experts in nuclear history have NO IDEA what actually happened on the subject of transmutations between 1907 and 1927 aside from the Rutherford/Blackett matter. The Le Matin article was more or less the tail end of it. There is a huge gap in history and textbooks of modern chemistry and physics that omitted this period. Your teachers probably don't know about it. Their teachers might have known about it. The research during this LOST HISTORY period was widely reported in popular media, such as the New York Times and Scientific American. Scientific papers were published in the top professional journals of the day, including Physical Review and Nature. Prominent scientists in the U.S., Europe, and Japan and even Nobel Prize winners were active in the research. However, by the 1930s, the entire body of research and results was dismissed as false and was left out of history books for nearly a century.
- Change made, as agreed I believe but please verify. Also I found ER's 1925 Royal Institution lecture on-line and added it as a source. Thanks for the discussions. Dirac66 (talk) 16:54, 29 June 2019 (UTC)
- Looks great. There's one other place in the next paragraph that says "complete reaction" which I think would be better said simply "reaction." I'll make that change, but if you disagree, please feel free to revert and discuss.
- I thank you as well for the discussions.
- StevenBKrivit (talk) 20:43, 29 June 2019 (UTC)
Incidence of cancer section
It seems to me that the section gives undue weight to something that was proved false. Also, it is not directly related to Rutherford himself, and its discussion should be moved to the Manchester University article. In any case, I think the section should be removed from here. --Ita140188 (talk) 20:57, 8 October 2019 (UTC)
Semi-protected edit request on 17 September 2020
This edit request to Ernest Rutherford has been answered. Set the |answered= or |ans= parameter to no to reactivate your request. |
Request to add Dalton Medal (1919) to 'Awards' in Infobox.
Request to add the following to be an Academic offices box:
Setonaery (talk) 08:54, 17 September 2020 (UTC)
Contribution to the age of the Earth
I came to this article to learn about Rutherford's contribution to the age of the Earth, e.g. what year he spoke on that topic to an audience that included Lord Kelvin. But the article is completely silent on what I had thought was a famous fact about Rutherford. Am I confused about this or what? Vaughan Pratt (talk) 21:53, 21 October 2020 (UTC)
- This lecture is mentioned in the article on Kelvin, see William Thomson, 1st Baron Kelvin#Age of the Earth: geology, paragraph 4. The year isn't quite specified in that article now, but the source article by England, Molnar and Richter says 1904. Perhaps the information should be included in this article on Rutherford. Dirac66 (talk) 22:49, 21 October 2020 (UTC)
- I have now added a mention in this article, with a link to the longer version in the Kelvin article. And I have put the date 1904 in both articles. Dirac66 (talk) 22:57, 10 November 2020 (UTC)
reaction notation
Besides the question of who was the first to generate a nuclear reaction and the first to properly identify the reaction products, I recently wondered in talk:proton about who was the first to use the reaction notation, similar to that used in chemistry? That is (reactants) arrow (products)? Gah4 (talk) 18:18, 11 February 2021 (UTC)
Semi-protected edit request on 13 March 2021
This edit request to Ernest Rutherford has been answered. Set the |answered= or |ans= parameter to no to reactivate your request. |
In the sixth paragraph of the "Scientific Research" section, the term "ionisation chamber" could be changed to "[ionization chamber]". That is all, thanks for reading. KomikoXVIII (talk) 00:26, 13 March 2021 (UTC)
 Partly done: MOS:ENGVAR for why we don't change the english variety; added the wikilink though. Cheers, RandomCanadian (talk / contribs) 00:49, 13 March 2021 (UTC)
Partly done: MOS:ENGVAR for why we don't change the english variety; added the wikilink though. Cheers, RandomCanadian (talk / contribs) 00:49, 13 March 2021 (UTC)
Semi-protected edit request on 9 May 2021
This edit request to Ernest Rutherford has been answered. Set the |answered= or |ans= parameter to no to reactivate your request. |
Please add the category Category:Peers created by George V. 2601:241:300:B610:2022:6C08:39EA:BAF1 (talk) 20:06, 9 May 2021 (UTC)
- Done. Dirac66 (talk) 22:55, 9 May 2021 (UTC)