Talk:E–Z notation
| This It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
Geocities
[edit]Although I'm not the original author who added the Geocities link, I'm unclear on why it was deleted. I've read through the webpage and it appears to give a more thorough, in-depth discussion of the EZ system than the quick, almost summary, overview presented in the WP article. JeramieHicks (talk) 19:46, 3 October 2008 (UTC)
- See WP:VERIFY for the kinds of sources used in the editing of science articles at Wikipedia. If the Geocities is a good read, then it also was cribbed from somewhere, and it is that authoritative somewhere that we want to read, and summarize, and cite here. Le Prof 50.179.252.14 (talk) 21:12, 2 March 2016 (UTC)
three of four
[edit]Currently,[1] the article says this notation can be used for double bonds with "three of four" substituents. Is that a typo for "three or four" or rather "up to four"? --Una Smith (talk) 02:26, 14 March 2009 (UTC)
- Fixed with "three or four". JeramieHicks (talk) 21:12, 8 April 2009 (UTC)
Edits of this date
[edit]Needed additions: The E-/Z- nomenclature applies not only to substituted alkenes, but also, applies (unequivocally) to any disubstituted system with an sp2-hybridized (e.g., element-element, e.g., carbon-carbon double) bond; hence, it is used routinely, even overwhelmingly, in the pedagogy of modern aldol reaction mechanisms, such as the Mukaiyama, to describe these enolate reactants. There is much discussion of aldols in Wikipedia, including with syn-/anti- product descriptions arising from E-/Z-enolates. Hence, images and text describing stereochemical cases other than alkenes/olefins are needed in this article. See for instance, Advanced Organic Chemistry. Part B: Reactions and Synthesis (5th ed.). Berlin, Germany: Springer Science & Business. 2010. pp. 64ff, esp. 67f and 98f, 82, 91, 88, 93. ISBN 0387683542. Retrieved 1 March 2016. {{cite book}}: Unknown parameter |authors= ignored (help), for a source to write these additions.
Cheers, Le Prof 50.179.252.14 (talk) 21:06, 2 March 2016 (UTC)
- Please also look at or add images of tri- and tetra-substituted cases—since the opening sentence explicitly mentions these—and then read through the prose for its general applicability to these cases as well. (As it stands, the article's text only makes sense for the simplest of all cases, that shown in the single image, and so again, the scope is too narrow for such a general article.) Le Prof 50.179.252.14 (talk) 21:20, 2 March 2016 (UTC)
'extension of cis/trans notation'
[edit]Combined with the example given, it would seem to imply that E would generally coincide with trans and Z with cis. At the very least, we need to give an example where this is not the case (e.g. 2-bromobut-2-ene, where the E isomer would be the cis isomer). (And we should really expand this beyond the basic cases.) Double sharp (talk) 04:11, 31 July 2016 (UTC)
- My thinking is that since the the complexity of E-Z notation comes from assigning priorities (as far as I know), we can just merge this article with Cahn–Ingold–Prelog priority rules and make complex examples that illustrate both CIP priority rules and the E-Z notation. (similar to the current examples for R/S assignments in the CIP priority rules article) GalobtterTalk to me! 08:03, 31 July 2016 (UTC)
E/Z vs cis trans
[edit]@DMacks and Michael D. Turnbull: Here's my thinking and my preliminary interpretation of March's discussion on the use of E/Z vs cis/trans. Two isomers of 2-butene exist, cis and trans. Unambiguous nomenclature. One doesnt need (or use) E/Z when the two substituents are the same. But when the two substituents are non-identical, then we turn to E/Z, E being sorta trans, and Z being sorta cis. That is the rationale for my test edit (I figured it would get a response). Maybe I am wrong. --Smokefoot (talk) 14:11, 5 December 2024 (UTC)
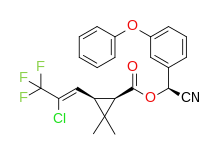
- Well, when the substituents are the same, you don't need R,S nomenclature at a single chiral carbon centre either, as it isn't chiral, so that's not a good argument. This article is about E–Z notation, so I think it needs to use that notation even in simple cases like 2-butene where the alternative (but not now IUPAC-preferred) cis/trans is unambiguous. Maybe we need a better example in the lead, such as 1,2,3,3,3-Pentafluoropropene where many beginner chemists would get the E or Z forms confused. Actually, my favourite teaching example is cyhalothrin, where the correct naming for the biologically active isomer involves (1R)-cis-Z. That's cis for the cyclopropane and Z for the double bond. Note that cis/trans nomenclature can be used for ring substituents whereas E/Z never is. So I agree with DMacks that the original was OK. Mike Turnbull (talk) 14:50, 5 December 2024 (UTC)
- Love that cyhalothrin example! I wonder why it defines absolute (R/S) of one of the two stereocenters of the cyclopropyl but then relative (cis/trans) of the other one to it, rather than each one's own R/S as in Deltamethrin's name? DMacks (talk) 16:10, 5 December 2024 (UTC)
- The deltamethrin-type name is right according to IUPAC's rules but IMO the use of the relative name is more useful. So perhaps I should not have used the word "correct" above. Mike Turnbull (talk) 16:32, 5 December 2024 (UTC)
- Love that cyhalothrin example! I wonder why it defines absolute (R/S) of one of the two stereocenters of the cyclopropyl but then relative (cis/trans) of the other one to it, rather than each one's own R/S as in Deltamethrin's name? DMacks (talk) 16:10, 5 December 2024 (UTC)

