Talk:Dibutyltin oxide
| This article is rated Start-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
Structure
[edit]
n-Bu2SnO is not a monomeric molecule as currently depicted in the article (File:Dibutyltinoxide.png, File:Dibutyltin-oxide-3D-spacefill.png). It's actually an insoluble polymer of some sort.
According to this 2002 review, "In solution most of the compounds (i.e. diorganotin oxides, [R2SnO]n) are four-coordinate as indicated by the 119Sn chemical shifts. Insoluble oxides such as Me2SnO or n-Bu2SnO, however, show chemical shifts that are considerably upfield shifted (−152 and −177 ppm, respectively) suggesting a higher coordination around tin in the solid-state for these types of compounds".

This 1987 paper is the original work the review cites. The authors tentatively propose a cross-linked network structure with cis-R2SnO3 tin coordination. I've reproduced their diagram on the right.
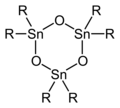
I should also note that with bulky R groups, diorganotin oxides adopt cyclic trimers or dimers. Several crystal structures have been reported, e.g. (tBu2SnO)3 from J. Organomet. Chem. (1984) 260, 271-280 shown below, and are discussed in the 2002 review.
This goes to show the importance of reading the literature on the structure of a chemical before drawing a picture of it. If you just guess, you'll eventually get it wrong and mislead our readers.