Streptococcus pyogenes
| Streptococcus pyogenes | |
|---|---|
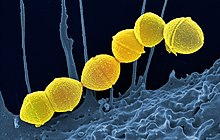
| |
| False-color scanning electron micrograph of chain of Streptococcus pyogenes bacteria (yellow) | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Bacillota |
| Class: | Bacilli |
| Order: | Lactobacillales |
| Family: | Streptococcaceae |
| Genus: | Streptococcus |
| Species: | S. pyogenes
|
| Binomial name | |
| Streptococcus pyogenes Rosenbach 1884
| |
Streptococcus pyogenes is a species of Gram-positive, aerotolerant bacteria in the genus Streptococcus. These bacteria are extracellular, and made up of non-motile and non-sporing cocci (round cells) that tend to link in chains. They are clinically important for humans, as they are an infrequent, but usually pathogenic, part of the skin microbiota that can cause group A streptococcal infection. S. pyogenes is the predominant species harboring the Lancefield group A antigen, and is often called group A Streptococcus (GAS). However, both Streptococcus dysgalactiae and the Streptococcus anginosus group can possess group A antigen as well. Group A streptococci, when grown on blood agar, typically produce small (2–3 mm) zones of beta-hemolysis, a complete destruction of red blood cells. The name group A (beta-hemolytic) Streptococcus is thus also used.[1]
The species name is derived from Greek words meaning 'a chain' (streptos) of berries (coccus [Latinized from kokkos]) and pus (pyo)-forming (genes), since a number of infections caused by the bacterium produce pus. The main criterion for differentiation between Staphylococcus spp. and Streptococcus spp. is the catalase test. Staphylococci are catalase positive whereas streptococci are catalase-negative.[2] S. pyogenes can be cultured on fresh blood agar plates. The PYR test allows for the differentiation of Streptococcus pyogenes from other morphologically similar beta-hemolytic streptococci (including S. dysgalactiae subsp. esquismilis) as S. pyogenes will produce a positive test result.[3]
An estimated 700 million GAS infections occur worldwide each year. While the overall mortality rate for these infections is less than 0.1%, over 650,000 of the cases are severe and invasive, and these cases have a mortality rate of 25%.[4] Early recognition and treatment are critical; diagnostic failure can result in sepsis and death.[5][6] S. pyogenes is clinically and historically significant as the cause of scarlet fever, which results from exposure to the species' exotoxin.[7]
Epidemiology
[edit]

Unlike most bacterial pathogens, S. pyogenes only infects humans. Thus, zoonotic transmission from an animal (or animal products) to a human is rare.[8]
S. pyogenes typically colonizes the throat, genital mucosa, rectum, and skin. Of healthy adults, 1% to 5% have throat, vaginal, or rectal carriage, with children being more common carriers. Most frequently, transmission from one person to another occurs due to inhalation of respiratory droplets, produced by sneezing and coughing from an infected person. Skin contact, contact with objects harboring the bacterium, and consumption of contaminated food are possible but uncommon modes of transmission. Streptococcal pharyngitis occurs most frequently in late winter to early spring in most countries as indoor spaces are used more often and thus more crowded. Disease cases are the lowest during autumn.[9]
Maternal S. pyogenes infection usually happens in late pregnancy, at more than 30 weeks of gestation to four weeks postpartum. Maternal infections account for 2 to 4% of all clinically diagnosed S. pyogenes infections.[9] The risk of sepsis is relatively high compared to other bacterial infections acquired during pregnancy, and S. pyogenes is a leading cause of septic shock and death in pregnant and postpartum women.[10]
Bacteriology
[edit]
Serotyping
[edit]In 1928, Rebecca Lancefield published a method for serotyping S. pyogenes based on its cell-wall polysaccharide,[11] a virulence factor displayed on its surface.[12] Later, in 1946, Lancefield described the serologic classification of S. pyogenes isolates based on components of their surface pili (known as the T-antigen)[13] which are used by bacteria to attach to host cells.[14] As of 2016, a total of 120 M proteins are identified. These M proteins are encoded by 234 types emm gene with greater than 1,200 alleles.[9]
Lysogeny
[edit]All strains of S. pyogenes are polylysogenized, in that they carry one or more bacteriophage on their genomes.[15] Some of the phages may be defective, but in some cases active phage may compensate for defects in others.[16] In general, the genome of S. pyogenes strains isolated during disease are >90% identical, they differ by the phage they carry.[17]
Virulence factors
[edit]S. pyogenes has several virulence factors that enable it to attach to host tissues, evade the immune response, and spread by penetrating host tissue layers.[18] A carbohydrate-based bacterial capsule composed of hyaluronic acid surrounds the bacterium, protecting it from phagocytosis by neutrophils.[2] In addition, the capsule and several factors embedded in the cell wall, including M protein, lipoteichoic acid, and protein F (SfbI) facilitate attachment to various host cells.[19] M protein also inhibits opsonization by the alternative complement pathway by binding to host complement regulators. The M protein found on some serotypes is also able to prevent opsonization by binding to fibrinogen.[2] However, the M protein is also the weakest point in this pathogen's defense, as antibodies produced by the immune system against M protein target the bacteria for engulfment by phagocytes. M proteins are unique to each strain, and identification can be used clinically to confirm the strain causing an infection.[20]
| Name | Description |
|---|---|
| Streptolysin O | An exotoxin, one of the bases of the organism's beta-hemolytic property, streptolysin O causes an immune response and detection of antibodies to it; antistreptolysin O (ASO) can be clinically used to confirm a recent infection. It is damaged by oxygen. |
| Streptolysin S | A cardiotoxic exotoxin, another beta-hemolytic component, not immunogenic and O2 stable: A potent cell poison affecting many types of cell including neutrophils, platelets, and subcellular organelles. |
| Streptococcal pyrogenic exotoxin A (SpeA) | Superantigens secreted by many strains of S. pyogenes: This streptococcal pyrogenic exotoxin is responsible for the rash of scarlet fever and many of the symptoms of streptococcal toxic shock syndrome, also known as toxic shock like syndrome (TSLS). |
| Streptococcal pyrogenic exotoxin C (SpeC) | |
| Streptococcal pyrogenic exotoxin B (SpeB) | A cysteine protease and the predominant secreted protein. Multiple actions, including degrading the extracellular matrix, cytokines, complement components, and immunoglobulins. Also called streptopain.[21] |
| Streptokinase | Enzymatically activates plasminogen, a proteolytic enzyme, into plasmin, which in turn digests fibrin and other proteins |
| Hyaluronidase | Hyaluronidase is widely assumed to facilitate the spread of the bacteria through tissues by breaking down hyaluronic acid, an important component of connective tissue. However, very few isolates of S. pyogenes are capable of secreting active hyaluronidase due to mutations in the gene that encodes the enzyme. Moreover, the few isolates capable of secreting hyaluronidase do not appear to need it to spread through tissues or to cause skin lesions.[22] Thus, the true role of hyaluronidase in pathogenesis, if any, remains unknown. |
| Streptodornase | Most strains of S. pyogenes secrete up to four different DNases, which are sometimes called streptodornase. The DNases protect the bacteria from being trapped in neutrophil extracellular traps (NETs) by digesting the NETs' web of DNA, to which are bound neutrophil serine proteases that can kill the bacteria.[23] |
| C5a peptidase | C5a peptidase cleaves a potent neutrophil chemotaxin called C5a, which is produced by the complement system.[24] C5a peptidase is necessary to minimize the influx of neutrophils early in infection as the bacteria are attempting to colonize the host's tissue.[25] C5a peptidase, although required to degrade the neutrophil chemotaxin C5a in the early stages of infection, is not required for S. pyogenes to prevent the influx of neutrophils as the bacteria spread through the fascia.[26] |
| Streptococcal chemokine protease | The affected tissue of patients with severe cases of necrotizing fasciitis are devoid of neutrophils.[27] The serine protease ScpC, which is released by S. pyogenes, is responsible for preventing the migration of neutrophils to the spreading infection. ScpC degrades the chemokine IL-8, which would otherwise attract neutrophils to the site of infection.[25][26] |
Genome
[edit]The genomes of different strains were sequenced (genome size is 1.8–1.9 Mbp)[28] encoding about 1700-1900 proteins (1700 in strain NZ131,[29][30] 1865 in strain MGAS5005[31][32]). Complete genome sequences of the type strain of S. pyogenes (NCTC 8198T = CCUG 4207T) are available in DNA Data Bank of Japan, European Nucleotide Archive, and GenBank under the accession numbers LN831034 and CP028841.[33]
Biofilm formation
[edit]Biofilms are a way for S. pyogenes, as well as other bacterial cells, to communicate with each other. In the biofilm gene expression for multiple purposes (such as defending against the host immune system) is controlled via quorum sensing.[34] One of the biofilm forming pathways in GAS is the Rgg2/3 pathway. It regulates SHP's (short hydrophobic peptides) that are quorum sensing pheromones a.k.a. autoinducers. The SHP's are translated to an immature form of the pheromone and must undergo processing, first by a metalloprotease enzyme inside the cell and then in the extracellular space, to reach their mature active form. The mode of transportation out of the cell and the extracellular processing factor(s) are still unknown. The mature SHP pheromone can then be taken into nearby cells and the cell it originated from via a transmembrane protein, oligopeptide permease.[34] In the cytosol the pheromones have two functions in the Rgg2/3 pathway. Firstly, they inhibit the activity of Rgg3 which is a transcriptional regulator repressing SHP production. Secondly, they bind another transcriptional regulator, Rgg2, that increases the production of SHP's, having an antagonistic effect to Rgg3. SHP's activating their own transcriptional activator creates a positive feedback loop, which is common for the production for quorum sensing peptides. It enables the rapid production of the pheromones in large quantities. The production of SHP's increases biofilm biogenesis.[34] It has been suggested that GAS switches between biofilm formation and degradation by utilizing pathways with opposing effects. Whilst Rgg2/3 pathway increases biofilm, the RopB pathway disrupts it. RopB is another Rgg-like protein (Rgg1) that directly activates SpeB (Streptococcal pyrogenic exotoxin B), a cysteine protease that acts as a virulence factor. In the absence of this pathway, biofilm formation is enhanced, possibly due to the lack of the protease degrading pheromones or other Rgg2/3 pathway counteracting effects.[34]
Disease
[edit]S. pyogenes is the cause of many human diseases, ranging from mild superficial skin infections to life-threatening systemic diseases.[2] The most frequent manifestations of disease are commonly known as scarlet fever. Infections typically begin in the throat or skin. The most striking sign is a strawberry-like rash. Examples of mild S. pyogenes infections include pharyngitis (strep throat) and localized skin infection (impetigo). Erysipelas and cellulitis are characterized by multiplication and lateral spread of S. pyogenes in deep layers of the skin. S. pyogenes invasion and multiplication in the fascia beneath the skin can lead to necrotizing fasciitis, a life-threatening surgical emergency.[35][36] The bacterium is also an important cause of infection in newborns, who are susceptible to some forms of the infection that are rarely seen in adults, including meningitis.[37][38]
Like many pathogenic bacteria, S. pyogenes may colonize a healthy person's respiratory system without causing disease, existing as a commensal member of the respiratory microbiota. It is commonly found in some populations as part of the mixed microbiome of the upper respiratory tract. Individuals who have the bacterium in their bodies but no signs of disease are known as asymptomatic carriers.[39][40][41] The bacteria may start to cause disease when the host's immune system weakens, such as during a viral respiratory infection, which may lead to S. pyogenes superinfection.[40][41]
S. pyogenes infections are commonly associated with the release of one or more bacterial toxins. The release of endotoxins from throat infections has been linked to the development of scarlet fever.[7] Other toxins produced by S. pyogenes may lead to streptococcal toxic shock syndrome, a life-threatening emergency.[2]
S. pyogenes can also cause disease in the form of post-infectious "non-pyogenic" (not associated with local bacterial multiplication and pus formation) syndromes. These autoimmune-mediated complications follow a small percentage of infections and include rheumatic fever and acute post-infectious glomerulonephritis. Both conditions appear several weeks following the initial streptococcal infection. Rheumatic fever is characterized by inflammation of the joints and/or heart following an episode of streptococcal pharyngitis. Acute glomerulonephritis, inflammation of the renal glomerulus, can follow streptococcal pharyngitis or skin infection.[citation needed]
S. pyogenes is sensitive to penicillin, and has not developed resistance to it,[42] making penicillin a suitable antibiotic to treat infections caused by this bacterium. Failure of treatment with penicillin is generally attributed to other local commensal microorganisms producing β-lactamase, or failure to achieve adequate tissue levels in the pharynx. Certain strains have developed resistance to macrolides, tetracyclines, and clindamycin.[43]
Vaccine
[edit]There is a polyvalent inactivated vaccine against several types of Streptococcus including S. pyogenes called " vacuna antipiogena polivalente BIOL" it is recommended an administration in a series of 5 weeks. Two weekly applications are made at intervals of 2 to 4 days. The vaccine is produced by the Instituto Biológico Argentino.[44]
There is another potential vaccine being developed; the vaccine candidate peptide is called StreptInCor.[45]
Applications
[edit]Bionanotechnology
[edit]Many S. pyogenes proteins have unique properties, which have been harnessed in recent years to produce a highly specific "superglue"[46][47] and a route to enhance the effectiveness of antibody therapy.[48]
Genome editing
[edit]The CRISPR system from this organism[49] that is used to recognize and destroy DNA from invading viruses, thus stopping the infection, was appropriated in 2012 for use as a genome-editing tool that could potentially alter any piece of DNA and later RNA.[50]
See also
[edit]References
[edit]- ^ "Streptococcus pyogenes - Pathogen Safety Data Sheets". Government of Canada, Public Health Agency of Canada. September 26, 2001.
- ^ a b c d e Ryan KJ, Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 978-0-8385-8529-0.
- ^ Spellerberg B, Brandt C (October 9, 2022) [Originally published September 15, 2022]. "Chapter 29: Laboratory Diagnosis of Streptococcus pyogenes (group A streptococci)". In Ferretti JJ, Stevens DL, Fischetti VA (eds.). Streptococcus pyogenes: Basic Biology to Clinical Manifestations (2nd ed.). Oklahoma City, United States: University of Oklahoma Health Sciences Center. PMID 36479747. Retrieved May 11, 2023 – via National Center for Biotechnology Information, National Library of Medicine.
- ^ Aziz RK, Kansal R, Aronow BJ, Taylor WL, Rowe SL, Kubal M, et al. (April 2010). Ahmed N (ed.). "Microevolution of group A streptococci in vivo: capturing regulatory networks engaged in sociomicrobiology, niche adaptation, and hypervirulence". PLOS ONE. 5 (4): e9798. Bibcode:2010PLoSO...5.9798A. doi:10.1371/journal.pone.0009798. PMC 2854683. PMID 20418946.
- ^ Jim Dwyer (July 11, 2012). "An Infection, Unnoticed, Turns Unstoppable". The New York Times. Retrieved July 12, 2012.
- ^ Jim Dwyer (July 18, 2012). "After Boy's Death, Hospital Alters Discharging Procedures". The New York Times. Retrieved July 19, 2012.
- ^ a b Pardo S, Perera TB (2023), "Scarlet Fever", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29939666, retrieved January 14, 2024
- ^ Gera K, McIver KS (October 2013). "Laboratory growth and maintenance of Streptococcus pyogenes (the Group A Streptococcus, GAS)". Current Protocols in Microbiology. 30: 9D.2.1–9D.2.13. doi:10.1002/9780471729259.mc09d02s30. PMC 3920295. PMID 24510893.
- ^ a b c Androulla E, Theresa L (February 10, 2016). "Epidemiology of Streptococcus pyogenes". Streptococcus pyogenes : Basic Biology to Clinical Manifestations. Oklahoma City, United States: University of Oklahoma Health Sciences Center. PMID 26866237. Retrieved February 24, 2018.
- ^ Tanaka H, Katsuragi S, Hasegawa J, Tanaka K, Osato K, Nakata M, et al. (January 2019). "The most common causative bacteria in maternal sepsis-related deaths in Japan were group A Streptococcus: A nationwide survey". Journal of Infection and Chemotherapy. 25 (1): 41–44. doi:10.1016/j.jiac.2018.10.004. PMID 30377069.
- ^ Pignanelli S, Brusa S, Pulcrano G, Catania MR, Cocchi E, Lanari M (October 2015). "A rare case of infant sepsis due to the emm-89 genotype of Group A Streptococcus within a community-acquired cluster". The New Microbiologica. 38 (4): 589–592. PMID 26485019.
- ^ Lancefield RC (January 1928). "The Antigenic Complex of Streptococcus Hæmolyticus". The Journal of Experimental Medicine. 47 (1): 91–103. doi:10.1084/jem.47.1.91. PMC 2131344. PMID 19869404.
- ^ Lancefield RC, Dole VP (October 1946). "The Properties of T Antigens Extracted from Group a Hemolytic Streptococci". The Journal of Experimental Medicine. 84 (5): 449–471. doi:10.1084/jem.84.5.449. PMC 2135665. PMID 19871581.
- ^ Mora M, Bensi G, Capo S, Falugi F, Zingaretti C, Manetti AG, et al. (October 2005). "Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens". Proceedings of the National Academy of Sciences of the United States of America. 102 (43): 15641–15646. Bibcode:2005PNAS..10215641M. doi:10.1073/pnas.0507808102. PMC 1253647. PMID 16223875.
- ^ Ferretti JJ, McShan WM, Ajdic D, Savic DJ, Savic G, Lyon K, et al. (April 2001). "Complete genome sequence of an M1 strain of Streptococcus pyogenes". Proceedings of the National Academy of Sciences of the United States of America. 98 (8): 4658–4663. Bibcode:2001PNAS...98.4658F. doi:10.1073/pnas.071559398. PMC 31890. PMID 11296296.
- ^ Canchaya C, Desiere F, McShan WM, Ferretti JJ, Parkhill J, Brüssow H (October 2002). "Genome analysis of an inducible prophage and prophage remnants integrated in the Streptococcus pyogenes strain SF370". Virology. 302 (2): 245–258. doi:10.1006/viro.2002.1570. PMID 12441069.
- ^ Banks DJ, Porcella SF, Barbian KD, Martin JM, Musser JM (December 2003). "Structure and distribution of an unusual chimeric genetic element encoding macrolide resistance in phylogenetically diverse clones of group A Streptococcus". The Journal of Infectious Diseases. 188 (12): 1898–1908. doi:10.1086/379897. PMID 14673771.
- ^ Patterson MJ (1996). "Streptococcus". In Baron S; et al. (eds.). Streptococcus. In: Baron's Medical Microbiology (4th ed.). University of Texas Medical Branch. ISBN 978-0-9631172-1-2.
- ^ Bisno AL, Brito MO, Collins CM (April 2003). "Molecular basis of group A streptococcal virulence". The Lancet. Infectious Diseases. 3 (4): 191–200. doi:10.1016/S1473-3099(03)00576-0. PMID 12679262.
- ^ Engel ME, Muhamed B, Whitelaw AC, Musvosvi M, Mayosi BM, Dale JB (February 2014). "Group A streptococcal emm type prevalence among symptomatic children in Cape Town and potential vaccine coverage". The Pediatric Infectious Disease Journal. 33 (2): 208–210. doi:10.1097/INF.0b013e3182a5c32a. PMC 3947201. PMID 23934204.
- ^ Nelson DC, Garbe J, Collin M (December 2011). "Cysteine proteinase SpeB from Streptococcus pyogenes - a potent modifier of immunologically important host and bacterial proteins". Biological Chemistry. 392 (12): 1077–1088. doi:10.1515/BC.2011.208. PMID 22050223. S2CID 207441558.
- ^ Starr CR, Engleberg NC (January 2006). "Role of hyaluronidase in subcutaneous spread and growth of group A streptococcus". Infection and Immunity. 74 (1): 40–48. doi:10.1128/IAI.74.1.40-48.2006. PMC 1346594. PMID 16368955.
- ^ Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, et al. (February 2006). "DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps". Current Biology. 16 (4): 396–400. Bibcode:2006CBio...16..396B. doi:10.1016/j.cub.2005.12.039. PMID 16488874. S2CID 667804.
- ^ Wexler DE, Chenoweth DE, Cleary PP (December 1985). "Mechanism of action of the group A streptococcal C5a inactivator". Proceedings of the National Academy of Sciences of the United States of America. 82 (23): 8144–8148. Bibcode:1985PNAS...82.8144W. doi:10.1073/pnas.82.23.8144. PMC 391459. PMID 3906656.
- ^ a b Ji Y, McLandsborough L, Kondagunta A, Cleary PP (February 1996). "C5a peptidase alters clearance and trafficking of group A streptococci by infected mice". Infection and Immunity. 64 (2): 503–510. doi:10.1128/IAI.64.2.503-510.1996. PMC 173793. PMID 8550199.
- ^ a b Hidalgo-Grass C, Mishalian I, Dan-Goor M, Belotserkovsky I, Eran Y, Nizet V, et al. (October 2006). "A streptococcal protease that degrades CXC chemokines and impairs bacterial clearance from infected tissues". The EMBO Journal. 25 (19): 4628–4637. doi:10.1038/sj.emboj.7601327. PMC 1589981. PMID 16977314.
- ^ Hidalgo-Grass C, Dan-Goor M, Maly A, Eran Y, Kwinn LA, Nizet V, et al. (February 2004). "Effect of a bacterial pheromone peptide on host chemokine degradation in group A streptococcal necrotising soft-tissue infections". Lancet. 363 (9410): 696–703. doi:10.1016/S0140-6736(04)15643-2. PMID 15001327. S2CID 7219898.
- ^ Beres SB, Richter EW, Nagiec MJ, Sumby P, Porcella SF, DeLeo FR, Musser JM (May 2006). "Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group A Streptococcus". Proceedings of the National Academy of Sciences of the United States of America. 103 (18): 7059–7064. Bibcode:2006PNAS..103.7059B. doi:10.1073/pnas.0510279103. PMC 1459018. PMID 16636287.
- ^ "Streptococcus pyogenes NZ131".
- ^ McShan WM, Ferretti JJ, Karasawa T, Suvorov AN, Lin S, Qin B, et al. (December 2008). "Genome sequence of a nephritogenic and highly transformable M49 strain of Streptococcus pyogenes". Journal of Bacteriology. 190 (23): 7773–7785. doi:10.1128/JB.00672-08. PMC 2583620. PMID 18820018.
- ^ Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, Ricklefs SM, et al. (September 2005). "Evolutionary origin and emergence of a highly successful clone of serotype M1 group a Streptococcus involved multiple horizontal gene transfer events". The Journal of Infectious Diseases. 192 (5): 771–782. doi:10.1086/432514. PMID 16088826.
- ^ "Streptococcus pyogenes MGAS5005".
- ^ Salvà-Serra F, Jaén-Luchoro D, Jakobsson HE, Gonzales-Siles L, Karlsson R, Busquets A, et al. (July 2020). "Complete genome sequences of Streptococcus pyogenes type strain reveal 100%-match between PacBio-solo and Illumina-Oxford Nanopore hybrid assemblies". Scientific Reports. 10 (1): 11656. doi:10.1038/s41598-020-68249-y. PMC 7363880. PMID 32669560.
- ^ a b c d Chang JC, LaSarre B, Jimenez JC, Aggarwal C, Federle MJ (August 2011). "Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development". PLOS Pathogens. 7 (8): e1002190. doi:10.1371/journal.ppat.1002190. PMC 3150281. PMID 21829369.
- ^ Schroeder JL, Steinke EE (December 2005). "Necrotizing fasciitis--the importance of early diagnosis and debridement". AORN Journal. 82 (6): 1031–1040. doi:10.1016/s0001-2092(06)60255-x. PMID 16478083.
- ^ "Necrotizing Fasciitis". CDC. Content source: National Center for Immunization and Respiratory Diseases, Division of Bacterial Diseases. Page maintained by: Office of the Associate Director for Communication, Digital Media Branch, Division of Public Affairs. October 26, 2017. Retrieved January 6, 2018.
- ^ Baucells BJ, Mercadal Hally M, Álvarez Sánchez AT, Figueras Aloy J (November 2016). "Asociaciones de probióticos para la prevención de la enterocolitis necrosante y la reducción de la sepsis tardía y la mortalidad neonatal en recién nacidos pretérmino de menos de 1.500g: una revisión sistemática" [Probiotic associations in the prevention of necrotising enterocolitis and the reduction of late-onset sepsis and neonatal mortality in preterm infants under 1,500g: A systematic review]. Anales de Pediatria (in Spanish). 85 (5): 247–255. doi:10.1016/j.anpedi.2015.07.038. PMID 26611880.
- ^ Berner R, Herdeg S, Gordjani N, Brandis M (July 2000). "Streptococcus pyogenes meningitis: report of a case and review of the literature". European Journal of Pediatrics. 159 (7): 527–529. doi:10.1007/s004310051325. PMID 10923229. S2CID 7693087.
- ^ Hung TY, Phuong LK, Grobler A, Tong SY, Freeth P, Pelenda A, Gibney KB, Steer AC (March 2024). "Antibiotics to eradicate Streptococcus pyogenes pharyngeal carriage in asymptomatic children and adults: A systematic review". J Infect. 88 (3): 106104. doi:10.1016/j.jinf.2024.01.003. PMID 38360357.
- ^ a b Othman AM, Assayaghi RM, Al-Shami HZ, Saif-Ali R (June 2019). "Asymptomatic carriage of Streptococcus pyogenes among school children in Sana'a city, Yemen". BMC Research Notes. 12 (1): 339. doi:10.1186/s13104-019-4370-5. PMC 6570875. PMID 31200755.
- ^ a b Oliver J, Malliya Wadu E, Pierse N, Moreland NJ, Williamson DA, Baker MG (March 2018). "Group A Streptococcus pharyngitis and pharyngeal carriage: A meta-analysis". PLOS Neglected Tropical Diseases. 12 (3): e0006335. doi:10.1371/journal.pntd.0006335. PMC 5875889. PMID 29554121.
- ^ Horn, D. L.; Zabriskie, J. B.; Austrian, R.; Cleary, P. P.; Ferretti, J. J.; Fischetti, V. A.; Gotschlich, E.; Kaplan, E. L.; McCarty, M.; Opal, S. M.; Roberts, R. B.; Tomasz, A.; Wachtfogel, Y. (June 1998). "Why have group A streptococci remained susceptible to penicillin? Report on a symposium". Clinical Infectious Diseases. 26 (6): 1341–1345. doi:10.1086/516375. ISSN 1058-4838. PMID 9636860.
- ^ Tadesse, Molla (March 2023). "Prevalence, Antibiotic Susceptibility Profile and Associated Factors of Group A Streptococcal pharyngitis Among Pediatric Patients with Acute Pharyngitis in Gondar, Northwest Ethiopia". Infection and Drug Resistance. 16: 1637–1648. doi:10.2147/IDR.S402292. PMC 40342. PMID 36992964.
- ^ "Package leaflet on BIOL official website" (PDF). Archived (PDF) from the original on October 10, 2022.
- ^ Guilherme L, Ferreira FM, Köhler KF, Postol E, Kalil J (February 2013). "A vaccine against Streptococcus pyogenes: the potential to prevent rheumatic fever and rheumatic heart disease". American Journal of Cardiovascular Drugs. 13 (1): 1–4. doi:10.1007/s40256-013-0005-8. PMID 23355360. S2CID 13071864.
- ^ Howarth M (May 2017). "Smart superglue in streptococci? The proof is in the pulling". The Journal of Biological Chemistry. 292 (21): 8998–8999. doi:10.1074/jbc.H117.777466. PMC 5448131. PMID 28550142.
- ^ Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, Howarth M (March 2012). "Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin". Proceedings of the National Academy of Sciences of the United States of America. 109 (12): E690–E697. Bibcode:2012PNAS..109E.690Z. doi:10.1073/pnas.1115485109. PMC 3311370. PMID 22366317.
- ^ Baruah K, Bowden TA, Krishna BA, Dwek RA, Crispin M, Scanlan CN (June 2012). "Selective deactivation of serum IgG: a general strategy for the enhancement of monoclonal antibody receptor interactions". Journal of Molecular Biology. 420 (1–2): 1–7. doi:10.1016/j.jmb.2012.04.002. PMC 3437440. PMID 22484364.
- ^ Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, et al. (March 2011). "CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III". Nature. 471 (7340): 602–607. Bibcode:2011Natur.471..602D. doi:10.1038/nature09886. PMC 3070239. PMID 21455174.
- ^ Zimmer C (June 3, 2016). "Scientists Find Form of Crispr Gene Editing With New Capabilities". The New York Times. ISSN 0362-4331. Retrieved June 10, 2016.
Further reading
[edit]- Freiberg JA, McIver KS, Shirtliff ME (September 2014). "In vivo expression of Streptococcus pyogenes immunogenic proteins during tibial foreign body infection". Infection and Immunity. 82 (9): 3891–3899. doi:10.1128/IAI.01831-14. PMC 4187806. PMID 25001603.
- Rosenbach FJ (1884). Mikro-Organismen bei den Wund-Infections-Krankheiten des Menschen (in German). J.F. Bergmann. OL 22886502M.
- Wilson LG (October 1987). "The early recognition of streptococci as causes of disease". Medical History. 31 (4): 403–414. doi:10.1017/s0025727300047268. PMC 1139783. PMID 3316876.
- Rolleston JD (November 1928). "The History of Scarlet Fever". British Medical Journal. 2 (3542): 926–929. doi:10.1136/bmj.2.3542.926. PMC 2456687. PMID 20774279.
- World Health Organization (2005). "The current evidence for the burden of group A streptococcal diseases". Archived from the original (PDF) on March 12, 2008. Retrieved August 22, 2011.
- Carapetis JR, Steer AC, Mulholland EK, Weber M (November 2005). "The global burden of group A streptococcal diseases". The Lancet. Infectious Diseases. 5 (11): 685–694. doi:10.1016/S1473-3099(05)70267-X. PMID 16253886. (corresponding summary article)
- Ferretti JJ, Stevens DL, Fischetti VA (2016). Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. Oklahoma City, OK: University of Oklahoma Health Sciences Center. PMID 26866208.
External links
[edit]- Type strain of Streptococcus pyogenes at BacDive - the Bacterial Diversity Metadatabase
- Nature-Inspired CRISPR Enzyme Discoveries Vastly Expand Genome Editing . On: SciTechDaily. June 16, 2020. Source: Media Lab, Massachusetts Institute of Technology.
