Semapimod
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| UNII |
|
| ChEMBL | |
| Chemical and physical data | |
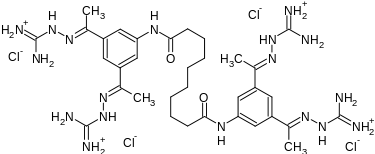
| Formula | C34H56Cl4N18O2 |
| Molar mass | 890.75 g·mol−1 |
| 3D model (JSmol) | |
| |
| | |
Semapimod (INN), formerly known as CNI-1493, is an investigational new drug which has anti-inflammatory,[1] anti-cytokine,[2] immunomodulatory,[3] antiviral[4] and antimalarial[5] properties.
History
[edit]Semapimod was developed at the former Picower Institute for Medical Research, and is now licensed to Cytokine PharmaSciences. In 2000, Cytokine PharmaSciences licensed anti-infective applications of semapimod to Axxima Pharmaceuticals, but Axxima became insolvent in Dec. 2004 and its assets were acquired by GPC Biotech, which has recently merged into Agennix AG. Although the disposition of Axxima's partial rights to semapimod was not specified in these merger announcements, Cytokine PharmaSciences does not currently list any licensees for semapimod on its website.[citation needed]
Mechanism of action
[edit]Semapimod was first developed to inhibit nitric oxide synthesis by inflammatory macrophages, via inhibition of the uptake of arginine which macrophages require for nitric oxide synthesis.[1] Subsequently, it was found that suppression of nitric oxide synthesis occurred even at semapimod concentrations 10-fold less than required for inhibition of arginine uptake, suggesting that this molecule was a more general inhibitor of inflammatory responses.[2] Further work revealed that semapimod suppressed the translation efficiency of tumor necrosis factor production.[6] Specifically, semapimod was found to be an inhibitor of p38 MAP kinase activation.[7] Surprisingly, however, the primary mode of action in vivo is now thought to be via stimulation of the vagus nerve, thereby down-regulating inflammatory pathways via the recently discovered cholinergic anti-inflammatory pathway.[8][9]
Pharmacology and clinical trials
[edit]In a preclinical study in rats, semapimod was found to suppress cytokine-storm induction by the anticancer cytokine interleukin-2 (IL-2) without decreasing its anticancer properties, allow larger doses of IL-2 to be administered.[10] A subsequent phase I trial in humans failed to show an increase in the tolerated dose of IL-2, although indications of pharmacological activity as an inhibitor of tumor necrosis factor production were observed.[11]
In a preliminary clinical trial of semapimod in patients with moderate to severe Crohn's disease, positive clinical changes were observed, including endoscopic improvement, positive responses in some patients not responding to infliximab, healing of fistulae, and indications for tapering of steroids; no significant adverse effects were observed.[12]
In a small clinical trial against post-ERCP pancreatitis, significant suppression was not observed, although investigators observed a significant reduction of the incidence of hyperamylasemia and the levels of post-ERCP amylase.[13]
In the clinical trials above, semapimod tetrahydrochloride was administered by intravenous injection. This route has drawbacks such as dose-limiting phlebitis. Recently Cytokine PharmaSciences has announced the development of novel salt forms of semapimod which are said to be orally absorbable; a phase I clinical trial of one of these salt forms, CPSI-2364, has been completed, and a phase II trial is planned for 2010.
Chemistry
[edit]Semapimod is synthesized by reacting 3,5-diacetylaniline[14] with sebacoyl chloride in the presence of pyridine, followed by reaction of the resulting tetraketone with aminoguanidine hydrochloride.[1]
References
[edit]- ^ a b c Bianchi M, Ulrich P, Bloom O, Meistrell M, Zimmerman GA, Schmidtmayerova H, et al. (March 1995). "An inhibitor of macrophage arginine transport and nitric oxide production (CNI-1493) prevents acute inflammation and endotoxin lethality". Molecular Medicine. 1 (3): 254–66. doi:10.1007/BF03401550. PMC 2229913. PMID 8529104.
- ^ a b Bianchi M, Bloom O, Raabe T, Cohen PS, Chesney J, Sherry B, et al. (March 1996). "Suppression of proinflammatory cytokines in monocytes by a tetravalent guanylhydrazone". The Journal of Experimental Medicine. 183 (3): 927–36. doi:10.1084/jem.183.3.927. PMC 2192362. PMID 8642296.
- ^ Martiney JA, Rajan AJ, Charles PC, Cerami A, Ulrich PC, Macphail S, et al. (June 1998). "Prevention and treatment of experimental autoimmune encephalomyelitis by CNI-1493, a macrophage-deactivating agent" (Free full text). Journal of Immunology. 160 (11): 5588–95. doi:10.4049/jimmunol.160.11.5588. PMID 9605164. S2CID 12503616.
- ^ Hauber I, Bevec D, Heukeshoven J, Krätzer F, Horn F, Choidas A, et al. (January 2005). "Identification of cellular deoxyhypusine synthase as a novel target for antiretroviral therapy". The Journal of Clinical Investigation (Free full text). 115 (1): 76–85. doi:10.1172/JCI21949. PMC 539192. PMID 15630446.
- ^ Specht S, Sarite SR, Hauber I, Hauber J, Görbig UF, Meier C, et al. (May 2008). "The guanylhydrazone CNI-1493: an inhibitor with dual activity against malaria-inhibition of host cell pro-inflammatory cytokine release and parasitic deoxyhypusine synthase". Parasitology Research. 102 (6): 1177–84. doi:10.1007/s00436-008-0891-x. PMID 18256853. S2CID 27771876.
- ^ Cohen PS, Nakshatri H, Dennis J, Caragine T, Bianchi M, Cerami A, Tracey KJ (April 1996). "CNI-1493 inhibits monocyte/macrophage tumor necrosis factor by suppression of translation efficiency". Proceedings of the National Academy of Sciences of the United States of America. 93 (9): 3967–71. Bibcode:1996PNAS...93.3967C. doi:10.1073/pnas.93.9.3967. PMC 39469. PMID 8632999.
- ^ Cohen PS, Schmidtmayerova H, Dennis J, Dubrovsky L, Sherry B, Wang H, et al. (May 1997). "The critical role of p38 MAP kinase in T cell HIV-1 replication". Molecular Medicine. 3 (5): 339–46. doi:10.1007/BF03401812. PMC 2230081. PMID 9205949.
- ^ Tracey KJ (February 2007). "Physiology and immunology of the cholinergic antiinflammatory pathway". The Journal of Clinical Investigation (Free full text). 117 (2): 289–96. doi:10.1172/JCI30555. PMC 1783813. PMID 17273548.
- ^ Oke SL, Tracey KJ (March 2008). "From CNI-1493 to the immunological homunculus: physiology of the inflammatory reflex" (Free full text). Journal of Leukocyte Biology. 83 (3): 512–7. CiteSeerX 10.1.1.527.419. doi:10.1189/jlb.0607363. PMID 18065685. S2CID 612157.
- ^ Kemeny MM, Botchkina GI, Ochani M, Bianchi M, Urmacher C, Tracey KJ (April 1998). "The tetravalent guanylhydrazone CNI-1493 blocks the toxic effects of interleukin-2 without diminishing antitumor efficacy". Proceedings of the National Academy of Sciences of the United States of America. 95 (8): 4561–6. Bibcode:1998PNAS...95.4561K. doi:10.1073/pnas.95.8.4561. PMC 22529. PMID 9539777.
- ^ Atkins MB, Redman B, Mier J, Gollob J, Weber J, Sosman J, et al. (March 2001). "A phase I study of CNI-1493, an inhibitor of cytokine release, in combination with high-dose interleukin-2 in patients with renal cancer and melanoma". Clinical Cancer Research. 7 (3): 486–92. PMID 11297238.
- ^ Hommes D, van den Blink B, Plasse T, Bartelsman J, Xu C, Macpherson B, et al. (January 2002). "Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn's disease". Gastroenterology. 122 (1): 7–14. doi:10.1053/gast.2002.30770. PMID 11781274.
- ^ van Westerloo DJ, Rauws EA, Hommes D, de Vos AF, van der Poll T, Powers BL, et al. (August 2008). "Pre-ERCP infusion of semapimod, a mitogen-activated protein kinases inhibitor, lowers post-ERCP hyperamylasemia but not pancreatitis incidence". Gastrointestinal Endoscopy. 68 (2): 246–54. doi:10.1016/j.gie.2008.01.034. PMID 18455169.
- ^ Ulrich P, Cerami A (January 1984). "Trypanocidal 1,3-arylene diketone bis(guanylhydrazone)s. Structure-activity relationships among substituted and heterocyclic analogues". Journal of Medicinal Chemistry. 27 (1): 35–40. doi:10.1021/jm00367a007. PMID 6690682.
