Rhomboid protease
| Rhomboid | |||||||||
|---|---|---|---|---|---|---|---|---|---|
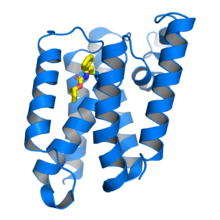 Escherichia coli rhomboid protease GlpG in complex with a beta-lactam inhibitor (yellow) bound to the catalytic serine residue. From PDB: 3ZMH.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Rhomboid | ||||||||
| Pfam | PF01694 | ||||||||
| Pfam clan | CL0207 | ||||||||
| InterPro | IPR002610 | ||||||||
| MEROPS | S54 | ||||||||
| SCOP2 | 144092 / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 165 | ||||||||
| OPM protein | 2ic8 | ||||||||
| |||||||||
The rhomboid proteases are a family of enzymes that exist in almost all species. They are proteases: they cut the polypeptide chain of other proteins. This proteolytic cleavage is irreversible in cells, and an important type of cellular regulation. Although proteases are one of the earliest and best studied class of enzyme, rhomboids belong to a much more recently discovered type: the intramembrane proteases. What is unique about intramembrane proteases is that their active sites are buried in the lipid bilayer of cell membranes, and they cleave other transmembrane proteins within their transmembrane domains.[2] About 30% of all proteins have transmembrane domains, and their regulated processing often has major biological consequences. Accordingly, rhomboids regulate many important cellular processes, and may be involved in a wide range of human diseases.
Intramembrane proteases
[edit]Rhomboids are intramembrane serine proteases.[3][4][5][6]: Abstract The other types of intramembrane protease are aspartyl- and metallo-proteases, respectively. The presenilins and signal peptide peptidase-like family, which are intramembrane aspartyl proteases, cleave substrates that include the Notch receptor and the amyloid precursor protein, which is implicated in Alzheimer's disease. The site-2 protease family, which are intramembrane metalloproteases, regulate among other things cholesterol biosynthesis and stress responses in bacteria. The different intramembrane protease families are evolutionarily and mechanistically unrelated, but there are clear common functional themes that link them. Rhomboids are perhaps the best characterised class.
History
[edit]Rhomboids were first named after a mutation in the fruit fly Drosophila, discovered in a famous genetic screen that led to a Nobel Prize for Christiane Nüsslein-Volhard and Eric Wieschaus.[7] In that screen they found a number of mutants with similar phenotypes: ‘pointy’ embryonic head skeletons.[6]: 192 They named them each with a pointy-themed name – one was rhomboid. At first this was noticed because a mutation disrupted development,[8]: 237 genetic analysis later proved that this group of genes were members of the epidermal growth factor (EGF) receptor signalling pathway,[9][10][6]: 192 [8]: abstract, 239 and that rhomboid was needed to generate the signal that activates the EGF receptor.[11][12][6]: 192 The molecular function of rhomboid took a bit longer to unravel but a combination of genetics and molecular techniques led to the discovery that Drosophila rhomboid[6]: 192, Fig 1 and other members of the family were the first known intramembrane serine proteases.[3]
Function
[edit]Rhomboids were first discovered as proteases that regulate EGF receptor signalling in Drosophila. By releasing the extracellular domain of the growth factor Spitz, from its transmembrane precursor, rhomboid triggers signalling.[3] Since then, many other important biological functions have been proposed.[6]: 196 [13]
- Later, Drosophilas' Rhomboid-1 was shown to regulate sleep, through a new function of an already-discovered mechanism.[6]: 201–2
- Although less well established than in Drosophila, there is some evidence that rhomboids may participate in growth factor signalling in mammals, including humans.[14][8]: 240, Mammalian Rhomboid Proteases They have also been implicated in ephrin signalling,[15] the cleavage of the anticoagulant protein thrombomodulin[16] and wound healing.[17]
- All eukaryotes have a mitochondrial rhomboid. In yeast this has been shown to control mitochondrial function and morphology by regulating membrane fusion via the cleavage of a dynamin-like GTPase called Mgm1p, the orthologue of human OPA1.[18][19] In Drosophila, the mitochondrial rhomboid (Rhomboid-7)[8]: 240–1, Mitochondrial Rhomboids also regulates mitochondrial membrane fusion.[20] Drosophila Opa1 and Rhomboid-7 appear to have the same relationship as in yeast.[6]: 201 In mammals too, mitochondrial function is disrupted in mutants of PARL, the mitochondrial rhomboid, but the range of functions is more complex. PARL regulates the remodelling of mitochondrial cristae,[21] is implicated in cell death[21] and metabolism,[22] and there is increasing evidence of an important role in Parkinson's disease;[23][24][25]
- Apicomplexan parasites (including Plasmodium, the agent that causes malaria, and Toxoplasma) rhomboids are used to reposition between attachment to a target cell and entry,[26]: 582, Figure 1 and most microneme[27]: 519 -produced adhesins are released from the microneme by rhomboids.[26]: 581 [28][29][30][31][32][33] Rhomboids have also been implicated in the pathogenicity of other parasites.[34] In Toxoplasma specifically, some serpins inhibit rhomboids.[27]: 519
- A rhomboid in the Gram-negative bacterium Providencia stuartii is required for the function of the twin-arginine protein translocation (TAT) machinery.[35]
- Rhomboids control EGF receptor signaling in Caenorhabditis elegans as in Drosophila.[6]: 201
Structure
[edit]Rhomboids were the first intramembrane proteases for which a high resolution crystal structure was solved.[36][37][38][39][40] These structures confirmed predictions that rhomboids have a core of six transmembrane domains, and that the catalytic site depends on a serine and histidine catalytic dyad. The structures also explained how a proteolytic reaction, which requires water molecules, can occur in the hydrophobic environment of a lipid bilayer: one of the central mysteries of intramembrane proteases.[41] The active site of rhomboid protease is in a hydrophilic indentation, in principle accessible to water from the bulk solution.[36][37][38][39][40] However, it has been proposed that there might be an auxiliary mechanism to facilitate access of water molecules to the catalytic dyad at the bottom of the active site to ensure catalytic efficiency.[42]
The active site of rhomboid protease is protected laterally from the lipid bilayer by its six constituent transmembrane helices, suggesting that substrate access to rhomboid active site is regulated. One area of uncertainty has been the route of substrate access. Substrates were initially proposed to enter between transmembrane segments (TMSs) 1 and 3,[36][39] but current evidence strongly supports an alternative access point, between TMSs 2 and 5.[37][38][40][43][44] This notion is also supported by the fact that mutations in TMS 5 have only a marginal effect on the thermodynamic stability of rhomboid, unlike other regions of the molecule.[45] Very recently, the first ever co-crystal structure of an intramembrane protease[46] – Escherichia coli's version of the rhomboid protease GlpG[8]: 239 – and a substrate-derived peptide bound in the active site[46] confirms and extends this substrate access model and provides implications for the mechanism of other rhomboid-superfamily proteins.[citation needed] E. coli's GlpG is unusual for its low enzyme/substrate binding affinity.[8]: 239 The details of how a substrate TMS may be recognized by rhomboid are however still unclear. Some authors propose that substrate access involves a large lateral displacement movement of TMS 5 to open up the core of rhomboid.[37][43] Other reports instead suggest that large lateral movement of TMS 5 is not required,[47] and propose that the surface of TMSs 2 and 5 rather serves as an "intramembrane exosite" mediating the recognition of substrate TMS.[46][48] The rhomboid ortholog in D. suzukii is Dsuz\DS10_00004507.[49]
Enzymatic specificity
[edit]Rhomboids do not cleave all transmembrane domains. In fact, they are highly specific, with a limited number of substrates. Most natural Rhomboid substrates known so far are type 1 single transmembrane domain proteins, with their amino termini in the luminal/extracellular compartment. However, recent studies suggested that type 2 membrane protein (i.e. with opposite topology: the amino terminus is cytoplasmic),[50] or even multipass membrane proteins could act as rhomboid substrates.[51] The specificity of rhomboids underlies their ability to control functions in a wide range of biological processes and, in turn, understanding what makes a particular transmembrane domain into a rhomboid substrate can shed light on rhomboid function in different contexts.
Initial work indicated that rhomboids recognise instability of the transmembrane alpha-helix at the site of cleavage as the main substrate determinant.[52] More recently, it has been found that rhomboid substrates are defined by two separable elements: the transmembrane domain and a primary sequence motif in or immediately adjacent to it.[48] This recognition motif directs where the substrate is cleaved, which can occur either within, or just outside, the transmembrane domain, in the juxtamembrane region.[48] In the former case helix destabilising residues downstream in substrate TMS are also necessary for efficient cleavage.[48] A detailed enzyme kinetics analysis has in fact shown that the recognition motif interactions with rhomboid active site determine the kcat of substrate cleavage.[53] The principles of substrate TMS recognition by rhomboid remain poorly understood, but numerous lines of evidence indicate that rhomboids (and perhaps also other intramembrane proteases) somehow recognise the structural flexibility or dynamics of transmembrane domain of their substrates.[42][54] Full appreciation of the biophysical and structural principles involved will require structural characterisation of the complex of rhomboid with the full transmembrane substrate.[55] As a first step towards this goal, a recent co-crystal structure of the enzyme in complex with a substrate-derived peptide containing mechanism-based inhibitor explains the observed recognition motif sequence preferences in rhomboid substrates structurally, and provides a significant advance in the current understanding of rhomboid specificity and mechanism of rhomboid-family proteins.[46]
In some Gram-negative bacteria, including Shewanella and Vibrio, up to thirteen proteins are found with GlyGly-CTERM, a C-terminal homology domain consisting of a glycine-rich motif, a highly hydrophobic transmembrane helix, and a cluster of basic residues. This domain appears to be the recognition sequence for rhombosortase, a branch of the rhomboid protease family limited to just those bacteria with the GlyGly-CTERM domain.[56]
Medical significance
[edit]The diversity of biological functions already known to depend on rhomboids is reflected in evidence that rhomboids play a role in a variety of diseases including cancer,[citation needed] parasite infection,[13] and diabetes.[citation needed] It is important to note, however, that there is no case yet established where a precise medical significance is fully validated.[6]
No drugs that modulate rhomboid activity have yet been reported, although a recent study has identified small molecule, mechanism-based inhibitors that could provide a basis for future drug development.[57]
The rhomboid-like family
[edit]Rhomboid proteases appear to be conserved in all eukaryotes and the vast majority of prokaryotes. Bioinformatic analysis highlights that some members of the rhomboid family lack the amino acid residues essential for proteolysis, implying that they cannot cleave substrates. These ‘pseudoproteases’ include a subfamily that have been named the iRhoms[58] (also known as RHBDF1 and RHBDF2). iRhoms can promote the ER associated degradation (ERAD) of EGF receptor ligands in Drosophila, thus providing a mechanism for regulating EGF receptor activity in the brain.[59] This implies that the fundamental cellular quality control mechanism is exploited by multicellular organisms to regulate signalling between cells. In mice, iRhoms are key trafficking chaperones required for the ER export of ADAM17/TACE and its maturation. iRhoms are thus required for the TNF-alpha and EGF receptor signalling, making them medically highly attractive.[59][60][61][62][63]
Phylogenetic analysis indicates that rhomboids are in fact members of a larger rhomboid-like superfamily or clan, which includes the derlin proteins, also involved in ERAD.[64]
Kinetoplastids have an unusually small rhomboid family repertoire, in Trypanosoma brucei XP 001561764 and XP 001561544, and in T. cruzi XP 805971, XP 802860, and XP 821055.[65]
Various rhomboid family proteins are vital to Toxoplasma gondii virulence and motility, including TgMIC2, TgMIC6, various AMA1 variants including TgAMA1, TgROM1, TgROM4, and TgROM5.[66]
Trypanosome mitochondria have TimRhom I and TimRhom II (two rhomboid family members with proteolytic function deactivated) in their presequence translocases. The difficulty in finding greater similarity either to eukaryotic or bacterial relatives may mean these came as part of the original mitochondrial progenitor.[67] Rhomboid-relatives may be membrane transport proteins in the ERAD and SELMA systems.[67]: 105
iRhoms
[edit]iRhoms are rhomboid-like proteins, but are not proteases. As with rhomboids they were first discovered in Drosophilae. To the contrary of rhomboids, however, iRhoms inhibit EGFr signaling. Knockout mice for iRhom2 have severe immune compromise.[8]: 243, iRhoms
References
[edit]- ^ Vinothkumar KR, Pierrat OA, Large JM, Freeman M (June 2013). "Structure of rhomboid protease in complex with β-lactam inhibitors defines the S2' cavity". Structure. 21 (6): 1051–8. doi:10.1016/j.str.2013.03.013. PMC 3690538. PMID 23665170.
- ^ Brown MS, Ye J, Rawson RB, Goldstein JL (February 2000). "Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans". Cell. 100 (4): 391–8. doi:10.1016/S0092-8674(00)80675-3. PMID 10693756. S2CID 12194770.
- ^ a b c Urban S, Lee JR, Freeman M (October 2001). "Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases". Cell. 107 (2): 173–82. doi:10.1016/s0092-8674(01)00525-6. PMID 11672525. S2CID 9026083.
- ^ Lemberg MK, Menendez J, Misik A, Garcia M, Koth CM, Freeman M (February 2005). "Mechanism of intramembrane proteolysis investigated with purified rhomboid proteases". The EMBO Journal. 24 (3): 464–72. doi:10.1038/sj.emboj.7600537. PMC 548647. PMID 15616571.
- ^ Urban S, Wolfe MS (February 2005). "Reconstitution of intramembrane proteolysis in vitro reveals that pure rhomboid is sufficient for catalysis and specificity". Proceedings of the National Academy of Sciences of the United States of America. 102 (6): 1883–8. Bibcode:2005PNAS..102.1883U. doi:10.1073/pnas.0408306102. PMC 548546. PMID 15684070.
- ^ a b c d e f g h i j Freeman M (2008). "Rhomboid proteases and their biological functions". Annual Review of Genetics. 42: 191–210. doi:10.1146/annurev.genet.42.110807.091628. PMID 18605900.
- ^ Jürgens G, Wieschaus E, Nüsslein-Volhard C, Kluding H (September 1984). "Mutations affecting the pattern of the larval cuticle inDrosophila melanogaster : II. Zygotic loci on the third chromosome". Wilhelm Roux's Archives of Developmental Biology. 193 (5): 283–295. doi:10.1007/BF00848157. PMID 28305338. S2CID 26608498.
- ^ a b c d e f g Freeman M (2014). "The rhomboid-like superfamily: molecular mechanisms and biological roles". Annual Review of Cell and Developmental Biology. 30: 235–54. doi:10.1146/annurev-cellbio-100913-012944. PMID 25062361. S2CID 31705365.
- ^ Sturtevant MA, Roark M, Bier E (June 1993). "The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway". Genes & Development. 7 (6): 961–73. doi:10.1101/gad.7.6.961. PMID 8504935.
- ^ Freeman M (October 1994). "The spitz gene is required for photoreceptor determination in the Drosophila eye where it interacts with the EGF receptor". Mechanisms of Development. 48 (1): 25–33. doi:10.1016/0925-4773(94)90003-5. PMID 7833286. S2CID 40396109.
- ^ Wasserman JD, Urban S, Freeman M (July 2000). "A family of rhomboid-like genes: Drosophila rhomboid-1 and roughoid/rhomboid-3 cooperate to activate EGF receptor signaling". Genes & Development. 14 (13): 1651–63. doi:10.1101/gad.14.13.1651. PMC 316740. PMID 10887159.
- ^ Bang AG, Kintner C (January 2000). "Rhomboid and Star facilitate presentation and processing of the Drosophila TGF-alpha homolog Spitz". Genes & Development. 14 (2): 177–86. doi:10.1101/gad.14.2.177. PMC 316351. PMID 10652272.
- ^ a b Urban S (June 2009). "Making the cut: central roles of intramembrane proteolysis in pathogenic microorganisms". Nature Reviews. Microbiology. 7 (6): 411–23. doi:10.1038/nrmicro2130. PMC 2818034. PMID 19421188.
- ^ Adrain C, Strisovsky K, Zettl M, Hu L, Lemberg MK, Freeman M (May 2011). "Mammalian EGF receptor activation by the rhomboid protease RHBDL2". EMBO Reports. 12 (5): 421–7. doi:10.1038/embor.2011.50. PMC 3090019. PMID 21494248.
- ^ Pascall JC, Brown KD (April 2004). "Intramembrane cleavage of ephrinB3 by the human rhomboid family protease, RHBDL2". Biochemical and Biophysical Research Communications. 317 (1): 244–52. doi:10.1016/j.bbrc.2004.03.039. PMID 15047175.
- ^ Lohi O, Urban S, Freeman M (February 2004). "Diverse substrate recognition mechanisms for rhomboids; thrombomodulin is cleaved by Mammalian rhomboids". Current Biology. 14 (3): 236–41. doi:10.1016/j.cub.2004.01.025. PMID 14761657. S2CID 17760607.
- ^ Cheng TL, Wu YT, Lin HY, Hsu FC, Liu SK, Chang BI, et al. (December 2011). "Functions of rhomboid family protease RHBDL2 and thrombomodulin in wound healing". The Journal of Investigative Dermatology. 131 (12): 2486–94. doi:10.1038/jid.2011.230. PMID 21833011.
- ^ Herlan M, Vogel F, Bornhovd C, Neupert W, Reichert AS (July 2003). "Processing of Mgm1 by the rhomboid-type protease Pcp1 is required for maintenance of mitochondrial morphology and of mitochondrial DNA". The Journal of Biological Chemistry. 278 (30): 27781–8. doi:10.1074/jbc.m211311200. PMID 12707284.
- ^ McQuibban GA, Saurya S, Freeman M (May 2003). "Mitochondrial membrane remodelling regulated by a conserved rhomboid protease". Nature. 423 (6939): 537–41. Bibcode:2003Natur.423..537M. doi:10.1038/nature01633. PMID 12774122. S2CID 4398146.
- ^ McQuibban GA, Lee JR, Zheng L, Juusola M, Freeman M (May 2006). "Normal mitochondrial dynamics requires rhomboid-7 and affects Drosophila lifespan and neuronal function". Current Biology. 16 (10): 982–9. doi:10.1016/j.cub.2006.03.062. PMID 16713954. S2CID 18751418.
- ^ a b Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, et al. (July 2006). "Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling". Cell. 126 (1): 163–75. doi:10.1016/j.cell.2006.06.021. PMID 16839884. S2CID 6396519.
- ^ Civitarese AE, MacLean PS, Carling S, Kerr-Bayles L, McMillan RP, Pierce A, et al. (May 2010). "Regulation of skeletal muscle oxidative capacity and insulin signaling by the mitochondrial rhomboid protease PARL". Cell Metabolism. 11 (5): 412–26. doi:10.1016/j.cmet.2010.04.004. PMC 3835349. PMID 20444421.
- ^ Whitworth AJ, Lee JR, Ho VM, Flick R, Chowdhury R, McQuibban GA (2008). "Rhomboid-7 and HtrA2/Omi act in a common pathway with the Parkinson's disease factors Pink1 and Parkin". Disease Models & Mechanisms. 1 (2–3): 168–74, discussion 173. doi:10.1242/dmm.000109. PMC 2562193. PMID 19048081.
- ^ Deas E, Plun-Favreau H, Gandhi S, Desmond H, Kjaer S, Loh SH, et al. (March 2011). "PINK1 cleavage at position A103 by the mitochondrial protease PARL". Human Molecular Genetics. 20 (5): 867–79. doi:10.1093/hmg/ddq526. PMC 3033179. PMID 21138942.
- ^ Meissner C, Lorenz H, Weihofen A, Selkoe DJ, Lemberg MK (June 2011). "The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking". Journal of Neurochemistry. 117 (5): 856–67. doi:10.1111/j.1471-4159.2011.07253.x. PMID 21426348.
- ^ a b Bisio H, Soldati-Favre D (September 2019). "Signaling Cascades Governing Entry into and Exit from Host Cells by Toxoplasma gondii". Annual Review of Microbiology. 73 (1). Annual Reviews: 579–599. doi:10.1146/annurev-micro-020518-120235. PMID 31500539. S2CID 202405949.
- ^ a b McKerrow JH, Caffrey C, Kelly B, Loke P, Sajid M (2006). "Proteases in parasitic diseases". Annual Review of Pathology. 1 (1). Annual Reviews: 497–536. doi:10.1146/annurev.pathol.1.110304.100151. PMID 18039124.
- ^ Urban S, Freeman M (June 2003). "Substrate specificity of rhomboid intramembrane proteases is governed by helix-breaking residues in the substrate transmembrane domain". Molecular Cell. 11 (6): 1425–34. doi:10.1016/s1097-2765(03)00181-3. PMID 12820957.
- ^ Baker RP, Wijetilaka R, Urban S (October 2006). "Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stages of malaria". PLOS Pathogens. 2 (10): e113. doi:10.1371/journal.ppat.0020113. PMC 1599764. PMID 17040128.
- ^ O'Donnell RA, Hackett F, Howell SA, Treeck M, Struck N, Krnajski Z, et al. (September 2006). "Intramembrane proteolysis mediates shedding of a key adhesin during erythrocyte invasion by the malaria parasite". The Journal of Cell Biology. 174 (7): 1023–33. doi:10.1083/jcb.200604136. PMC 2064393. PMID 17000879.
- ^ Santos JM, Ferguson DJ, Blackman MJ, Soldati-Favre D (January 2011). "Intramembrane cleavage of AMA1 triggers Toxoplasma to switch from an invasive to a replicative mode". Science. 331 (6016): 473–7. Bibcode:2011Sci...331..473S. doi:10.1126/science.1199284. PMID 21205639. S2CID 26806264.
- ^ Srinivasan P, Coppens I, Jacobs-Lorena M (January 2009). "Distinct roles of Plasmodium rhomboid 1 in parasite development and malaria pathogenesis". PLOS Pathogens. 5 (1): e1000262. doi:10.1371/journal.ppat.1000262. PMC 2607553. PMID 19148267.
- ^ Lin JW, Meireles P, Prudêncio M, Engelmann S, Annoura T, Sajid M, et al. (April 2013). "Loss-of-function analyses defines vital and redundant functions of the Plasmodium rhomboid protease family". Molecular Microbiology. 88 (2): 318–38. doi:10.1111/mmi.12187. PMID 23490234.
- ^ Baxt LA, Baker RP, Singh U, Urban S (June 2008). "An Entamoeba histolytica rhomboid protease with atypical specificity cleaves a surface lectin involved in phagocytosis and immune evasion". Genes & Development. 22 (12): 1636–46. doi:10.1101/gad.1667708. PMC 2428061. PMID 18559479.
- ^ Stevenson LG, Strisovsky K, Clemmer KM, Bhatt S, Freeman M, Rather PN (January 2007). "Rhomboid protease AarA mediates quorum-sensing in Providencia stuartii by activating TatA of the twin-arginine translocase". Proceedings of the National Academy of Sciences of the United States of America. 104 (3): 1003–8. Bibcode:2007PNAS..104.1003S. doi:10.1073/pnas.0608140104. PMC 1783354. PMID 17215357.
- ^ a b c Wang Y, Zhang Y, Ha Y (November 2006). "Crystal structure of a rhomboid family intramembrane protease". Nature. 444 (7116): 179–80. Bibcode:2006Natur.444..179W. doi:10.1038/nature05255. PMID 17051161. S2CID 4350345.
- ^ a b c d Wu Z, Yan N, Feng L, Oberstein A, Yan H, Baker RP, et al. (December 2006). "Structural analysis of a rhomboid family intramembrane protease reveals a gating mechanism for substrate entry". Nature Structural & Molecular Biology. 13 (12): 1084–91. doi:10.1038/nsmb1179. PMID 17099694. S2CID 8308111.
- ^ a b c Ben-Shem A, Fass D, Bibi E (January 2007). "Structural basis for intramembrane proteolysis by rhomboid serine proteases". Proceedings of the National Academy of Sciences of the United States of America. 104 (2): 462–6. Bibcode:2007PNAS..104..462B. doi:10.1073/pnas.0609773104. PMC 1766407. PMID 17190827.
- ^ a b c Lemieux MJ, Fischer SJ, Cherney MM, Bateman KS, James MN (January 2007). "The crystal structure of the rhomboid peptidase from Haemophilus influenzae provides insight into intramembrane proteolysis". Proceedings of the National Academy of Sciences of the United States of America. 104 (3): 750–4. Bibcode:2007PNAS..104..750L. doi:10.1073/pnas.0609981104. PMC 1783385. PMID 17210913.
- ^ a b c Vinothkumar KR (March 2011). "Structure of rhomboid protease in a lipid environment". Journal of Molecular Biology. 407 (2): 232–47. doi:10.1016/j.jmb.2011.01.029. PMC 3093617. PMID 21256137.
- ^ Lemberg MK, Freeman M (December 2007). "Cutting proteins within lipid bilayers: rhomboid structure and mechanism". Molecular Cell. 28 (6): 930–40. doi:10.1016/j.molcel.2007.12.003. PMID 18158892.
- ^ a b Moin SM, Urban S (November 2012). "Membrane immersion allows rhomboid proteases to achieve specificity by reading transmembrane segment dynamics". eLife. 1: e00173. doi:10.7554/eLife.00173. PMC 3494066. PMID 23150798.
- ^ a b Baker RP, Young K, Feng L, Shi Y, Urban S (May 2007). "Enzymatic analysis of a rhomboid intramembrane protease implicates transmembrane helix 5 as the lateral substrate gate". Proceedings of the National Academy of Sciences of the United States of America. 104 (20): 8257–62. doi:10.1073/pnas.0700814104. PMC 1895938. PMID 17463085.
- ^ Wang Y, Maegawa S, Akiyama Y, Ha Y (December 2007). "The role of L1 loop in the mechanism of rhomboid intramembrane protease GlpG". Journal of Molecular Biology. 374 (4): 1104–13. doi:10.1016/j.jmb.2007.10.014. PMC 2128867. PMID 17976648.
- ^ Baker RP, Urban S (September 2012). "Architectural and thermodynamic principles underlying intramembrane protease function". Nature Chemical Biology. 8 (9): 759–68. doi:10.1038/nchembio.1021. PMC 4028635. PMID 22797666.
- ^ a b c d Zoll S, Stanchev S, Began J, Skerle J, Lepšík M, Peclinovská L, Majer P, Strisovsky K (October 2014). "Substrate binding and specificity of rhomboid intramembrane protease revealed by substrate-peptide complex structures". The EMBO Journal. 33 (20): 2408–21. doi:10.15252/embj.201489367. PMC 4253528. PMID 25216680.
- ^ Xue Y, Ha Y (June 2013). "Large lateral movement of transmembrane helix S5 is not required for substrate access to the active site of rhomboid intramembrane protease". The Journal of Biological Chemistry. 288 (23): 16645–54. doi:10.1074/jbc.M112.438127. PMC 3675599. PMID 23609444.
- ^ a b c d Strisovsky K, Sharpe HJ, Freeman M (December 2009). "Sequence-specific intramembrane proteolysis: identification of a recognition motif in rhomboid substrates". Molecular Cell. 36 (6): 1048–59. doi:10.1016/j.molcel.2009.11.006. PMC 2941825. PMID 20064469.
- ^ "FlyBase Gene Report: Dmel\rho". FlyBase. 2021-04-13. Retrieved 2021-06-08. Larkin A, Marygold SJ, Antonazzo G, Attrill H, Dos Santos G, Garapati PV, et al. (January 2021). "FlyBase: updates to the Drosophila melanogaster knowledge base". Nucleic Acids Research. 49 (D1): D899 – D907. doi:10.1093/nar/gkaa1026. PMC 7779046. PMID 33219682.
- ^ Tsruya R, Wojtalla A, Carmon S, Yogev S, Reich A, Bibi E, et al. (March 2007). "Rhomboid cleaves Star to regulate the levels of secreted Spitz". The EMBO Journal. 26 (5): 1211–20. doi:10.1038/sj.emboj.7601581. PMC 1817629. PMID 17304216.
- ^ Fleig L, Bergbold N, Sahasrabudhe P, Geiger B, Kaltak L, Lemberg MK (August 2012). "Ubiquitin-dependent intramembrane rhomboid protease promotes ERAD of membrane proteins". Molecular Cell. 47 (4): 558–69. doi:10.1016/j.molcel.2012.06.008. PMID 22795130.
- ^ Akiyama Y, Maegawa S (May 2007). "Sequence features of substrates required for cleavage by GlpG, an Escherichia coli rhomboid protease". Molecular Microbiology. 64 (4): 1028–37. doi:10.1111/j.1365-2958.2007.05715.x. PMID 17501925. S2CID 33930463.
- ^ Dickey SW, Baker RP, Cho S, Urban S (December 2013). "Proteolysis inside the membrane is a rate-governed reaction not driven by substrate affinity". Cell. 155 (6): 1270–81. doi:10.1016/j.cell.2013.10.053. PMC 3917317. PMID 24315097.
- ^ Langosch D, Scharnagl C, Steiner H, Lemberg MK (June 2015). "Understanding intramembrane proteolysis: from protein dynamics to reaction kinetics". Trends in Biochemical Sciences. 40 (6): 318–27. doi:10.1016/j.tibs.2015.04.001. PMID 25941170.
- ^ Strisovsky K (April 2013). "Structural and mechanistic principles of intramembrane proteolysis--lessons from rhomboids". The FEBS Journal. 280 (7): 1579–603. doi:10.1111/febs.12199. PMID 23432912. S2CID 6316872.
- ^ Haft DH, Varghese N (2011). "GlyGly-CTERM and rhombosortase: a C-terminal protein processing signal in a many-to-one pairing with a rhomboid family intramembrane serine protease". PLOS ONE. 6 (12): e28886. Bibcode:2011PLoSO...628886H. doi:10.1371/journal.pone.0028886. PMC 3237569. PMID 22194940.
- ^ Pierrat OA, Strisovsky K, Christova Y, Large J, Ansell K, Bouloc N, Smiljanic E, Freeman M (April 2011). "Monocyclic β-lactams are selective, mechanism-based inhibitors of rhomboid intramembrane proteases". ACS Chemical Biology. 6 (4): 325–35. doi:10.1021/cb100314y. PMC 3077804. PMID 21175222.
- ^ Lemberg MK, Freeman M (November 2007). "Functional and evolutionary implications of enhanced genomic analysis of rhomboid intramembrane proteases". Genome Research. 17 (11): 1634–46. doi:10.1101/gr.6425307. PMC 2045146. PMID 17938163.
- ^ a b Zettl M, Adrain C, Strisovsky K, Lastun V, Freeman M (April 2011). "Rhomboid family pseudoproteases use the ER quality control machinery to regulate intercellular signaling". Cell. 145 (1): 79–91. doi:10.1016/j.cell.2011.02.047. PMC 3149277. PMID 21439629.
- ^ Adrain C, Zettl M, Christova Y, Taylor N, Freeman M (January 2012). "Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE". Science. 335 (6065): 225–8. Bibcode:2012Sci...335..225A. doi:10.1126/science.1214400. PMC 3272371. PMID 22246777.
- ^ McIlwain DR, Lang PA, Maretzky T, Hamada K, Ohishi K, Maney SK, et al. (January 2012). "iRhom2 regulation of TACE controls TNF-mediated protection against Listeria and responses to LPS". Science. 335 (6065): 229–32. Bibcode:2012Sci...335..229M. doi:10.1126/science.1214448. PMC 4250273. PMID 22246778.
- ^ Christova Y, Adrain C, Bambrough P, Ibrahim A, Freeman M (October 2013). "Mammalian iRhoms have distinct physiological functions including an essential role in TACE regulation". EMBO Reports. 14 (10): 884–90. doi:10.1038/embor.2013.128. PMC 3807218. PMID 23969955.
- ^ Li X, Maretzky T, Weskamp G, Monette S, Qing X, Issuree PD, et al. (May 2015). "iRhoms 1 and 2 are essential upstream regulators of ADAM17-dependent EGFR signaling". Proceedings of the National Academy of Sciences of the United States of America. 112 (19): 6080–5. Bibcode:2015PNAS..112.6080L. doi:10.1073/pnas.1505649112. PMC 4434755. PMID 25918388.
- ^ "Clan: Rhomboid-like (CL0207)". Pfam.
- ^ M Santos J, Graindorge A, Soldati-Favre D (2012). "New insights into parasite rhomboid proteases". Molecular and Biochemical Parasitology. 182 (1–2). Elsevier: 27–36. doi:10.1016/j.molbiopara.2011.11.010. PMID 22173057.
- ^ Dogga SK, Soldati-Favre D (December 2016). "Biology of rhomboid proteases in infectious diseases". Seminars in Cell & Developmental Biology. 60. Elsevier: 38–45. doi:10.1016/j.semcdb.2016.08.020. PMID 27567708. S2CID 34820332. p. 41:
2.3.1
- ^ a b Harsman A, Schneider A (February 2017). "Mitochondrial protein import in trypanosomes: Expect the unexpected". Traffic. 18 (2). Wiley-Blackwell: 96–109. doi:10.1111/tra.12463. PMID 27976830. S2CID 206334512.: 103
Further reading
[edit]External links
[edit]- "Summary for family S54 (Rhomboid family)". MEROPS.
- "EC 3.4.21.105". Expasy. SIB Swiss Institute of Bioinformatics.
