Endometriosis
| Endometriosis | |
|---|---|
 | |
| Endometriosis as seen during laparoscopic surgery | |
| Specialty | Gynecology |
| Symptoms | Pelvic pain, infertility[1] |
| Usual onset | Unknown. First symptoms appear at the age before 20–30 years old.[2][3][4] |
| Duration | Long term[1] |
| Causes | Unknown[1] |
| Risk factors | Family history[3] |
| Diagnostic method | Based on symptoms, medical imaging, tissue biopsy[3] |
| Differential diagnosis | Pelvic inflammatory disease, irritable bowel syndrome, interstitial cystitis, fibromyalgia[1] |
| Prevention | Combined birth control pills, exercise, avoiding alcohol and caffeine[3] |
| Treatment | NSAIDs, continuous birth control pills, intrauterine device with progestogen, surgery[3] |
| Frequency | 10–15% of all women of reproductive age[5] |
| Deaths | ≈100 (0.0 to 0.1 per 100,000, 2015)[6][7] |
Endometriosis is a disease in which cells like those in the endometrium, the layer of tissue that normally covers the inside of the uterus, grow outside the uterus.[8][9] It occurs in humans and a limited number of menstruating mammals. Lesions can be found on ovaries, fallopian tubes, tissue around the uterus and ovaries (peritoneum), intestines, bladder, and diaphragm; and may also occur in other parts of the body.[3] Symptoms include pelvic pain, heavy and painful periods, pain with bowel movements, painful urination, pain during sexual intercourse and infertility.[1][10] Nearly half of those affected have chronic pelvic pain, while 70% feel pain during menstruation.[1] Up to half of affected individuals are infertile.[1] About 25% of individuals have no symptoms and 85% of those seen with infertility in a tertiary center have no pain.[1][11] Endometriosis can have both social and psychological effects.[12]
Diagnosis is usually based on symptoms and medical imaging;[3] however, a definitive diagnosis is made through laparoscopy and biopsy.[3] Other causes of similar symptoms include pelvic inflammatory disease, irritable bowel syndrome, interstitial cystitis, and fibromyalgia.[1] Endometriosis is often misdiagnosed and many patients report being incorrectly told their symptoms are trivial or normal.[12] Patients with endometriosis see an average of seven physicians before receiving a correct diagnosis, with an average delay of 6.7 years between the onset of symptoms and surgically obtained biopsies, the gold standard for diagnosing the condition. This places endometriosis at the extreme end of diagnostic inefficiency.[13]
Nearly 11 million women were affected by endometriosis, globally, in 2015.[6] Other sources estimate 6 to 10% of the general female population might have endometriosis.[1] Ethnic differences have been observed in endometriosis, as Southeast Asian and East Asian women are significantly more likely than White women to be diagnosed with endometriosis.[14][15]
The main cause of endometriosis is exposure to elevated levels of the female sex hormone estrogen, as well as estrogen receptor sensitivity.[16] Estrogen exposure worsens the inflammatory symptoms of endometriosis by stimulating an immune response.[17][18]
While there is no cure for endometriosis, several treatments may improve symptoms.[1] This may include pain medication, hormonal treatments or surgery.[3] The recommended pain medication is usually a non-steroidal anti-inflammatory drug (NSAID), such as naproxen.[3] Taking the active component of the birth control pill continuously or using an intrauterine device with progestogen may also be useful.[3] Gonadotropin-releasing hormone agonist (GnRH agonist) may improve the ability of those who are infertile to conceive.[3] Surgical removal of endometriosis may be used to treat those whose symptoms are not manageable with other treatments.[3]
Signs and symptoms
[edit]
Pain and infertility are common symptoms, although 20–25% of affected women are asymptomatic.[1] Presence of pain symptoms are associated with the type of endometrial lesions as 50% of women with typical lesions, 10% of women with cystic ovarian lesions, and 5% of women with deep endometriosis do not have pain.[19]
Pelvic pain
[edit]A major symptom of endometriosis is recurring pelvic pain. The pain can range from mild to severe cramping or stabbing pain that occurs on both sides of the pelvis, in the lower back and rectal area, and even down the legs. The amount of pain a person feels correlates weakly with the extent or stage (1 through 4) of endometriosis, with some individuals having little or no pain despite having extensive endometriosis or endometriosis with scarring, while others may have severe pain even though they have only a few small areas of endometriosis.[20] The most severe pain is typically associated with menstruation. Pain can also start a week before a menstrual period, during and even a week after a menstrual period, or it can be constant. The pain can be debilitating and result in emotional stress.[21] Symptoms of endometriosis-related pain may include:
- Dysmenorrhea (64%)[22] – painful, sometimes disabling cramps during the menstrual period; pain may get worse over time (progressive pain), also lower back pains linked to the pelvis
- Chronic pelvic pain – typically accompanied by lower back pain or abdominal pain
- Dyspareunia – painful sexual intercourse
- Dysuria – urinary urgency, frequency, and sometimes painful voiding[23]
- Mittelschmerz – pain associated with ovulation[24]
- Bodily movement pain – present during exercise, standing, or walking[23]
Compared with patients with superficial endometriosis, those with deep disease appear to be more likely to report shooting rectal pain and a sense of their insides being pulled down.[25] Individual pain areas and pain intensity appear to be unrelated to the surgical diagnosis, and the area of pain unrelated to the area of endometriosis.[25]
There are multiple causes of pain. Endometriosis lesions react to hormonal stimulation and may "bleed" during menstruation. The blood accumulates locally if it is not cleared shortly by the immune, circulatory, and lymphatic systems. This accumulation can lead to swelling, which triggers inflammation with the activation of cytokines, resulting in pain. Another source of pain is organ dislocation that arises from adhesion binding internal organs together. The ovaries, the uterus, the oviducts, the peritoneum, and the bladder can all be bound together. Pain triggered in this way can last throughout the menstrual cycle, not just during menstrual periods.[26]
Additionally, endometriotic lesions can develop their own nerve supply, creating a direct and two-way interaction between lesions and the central nervous system. This interaction can produce a variety of individual differences in pain that, in some cases, become independent of the disease itself.[20] Nerve fibers and blood vessels are thought to grow into endometriosis lesions by a process known as neuroangiogenesis.[27]
Infertility
[edit]About a third of women with infertility have endometriosis.[1] Among those with endometriosis, about 40% are infertile.[1] The pathogenesis of infertility varies by disease stage: in early-stage disease, it is hypothesised to result from an inflammatory response that impairs various aspects of conception, whereas in later stages, distorted pelvic anatomy and adhesions contribute to impaired fertilisation.[28]
Other
[edit]Bowel endometriosis may include symptoms like diarrhea, constipation,tenesmus, dyschezia, and, rarely, rectal bleeding. Other symptoms include chronic fatigue, nausea and vomiting, migraines, low-grade fevers, heavy (44%) and/or irregular periods (60%), and hypoglycemia.[22][29][23] Endometriosis is associated with certain types of cancers, notably some types of ovarian cancer,[30] non-Hodgkin's lymphoma and brain cancer.[31] Endometriosis is however unrelated to endometrial cancer.[32]
Rarely, endometriosis can cause endometrium-like tissue to be found in other parts of the body. Thoracic endometriosis occurs when endometrium-like tissue implants in the lungs or pleura. Manifestations of this include coughing up blood, a collapsed lung, or bleeding into the pleural space.[14][33] Endometriosis may also affect the nearby colon which in rare situations may progress to partial obstruction requiring emergency surgery.[34]
Stress may be a contributing factor or a consequence of endometriosis.[35]
Complications
[edit]Physical health
[edit]Complications of endometriosis include internal scarring, adhesions, pelvic cysts, ovarian chocolate cysts, ruptured cysts, and bowel and ureter obstruction resulting from pelvic adhesions.[36] Endometriosis-associated infertility may result from scar formation and anatomical distortions caused by the condition.[3]
Ovarian endometriosis may complicate pregnancy through decidualization, abscess formation and/or rupture.[37]
Thoracic endometriosis can be associated with recurrent thoracic endometriosis syndrome which manifests during menstrual periods. It includes catamenial pneumothorax in 73% of women, catamenial hemothorax in 14%, catamenial hemoptysis in 7%, and pulmonary nodules in 6%.[38][39]
A 20-year study involving 12,000 women with endometriosis found that individuals under 40 are three times more likely to develop heart problems compared to their healthy peers.[40]
Results of a 30-year study of reproductive and pregnancy outcomes, which included over 14,000+ women of child-bearing age, were presented at the 2015 European Society of Human Reproduction and Embryology (ESHRE) annual congress.[41] The study indicated that 39% of women with surgically confirmed non-graded endometriosis had a 270% higher risk for ectopic pregnancy and a 76% higher risk for miscarriage compared to their peers. For women with deep endometriosis (>5 mm invasion, ASRM Stage II and higher), the risk of miscarriage increased by 298%.[42]
Women with endometriosis also face a significantly increased risk of experiencing ante- and postpartum hemorrhage[41] as well as a 170% increased risk of severe pre-eclampsia[43] during pregnancy.
Endometriosis slightly increases the risk (about 1% or less) of developing ovarian, breast and thyroid cancers compared to women without the condition.[44]
The mortality rates associated with endometriosis are low, with unadjusted and age-standardized death rates of 0.1 and 0.0 per 100,000, respectively.[6]
Sciatic endometriosis also called catamenial or cyclical sciatica ,is a rare form where endometriosis affects the sciatic nerve. Diagnosis is usually confirmed through MRI or CT-myelography.[45]
Endometriosis can also impact a woman's fetus or neonate, increasing the risks for congenital malformations, preterm delivery and higher neonatal death rates.[43]
Mental health
[edit]"Endometriosis is associated with an elevated risk of developing depression and anxiety disorders".[46] Studies suggest this is partially due to the pelvic pain experienced by endometriosis patients.
"It has been demonstrated that pelvic pain has significant negative effects on women's mental health and quality of life; in particular, women who suffer from pelvic pain report high levels of anxiety and depression, loss of working ability, limitations in social activities and a poor quality of life" [47]
Risk factors
[edit]Genetics
[edit]Endometriosis is a heritable condition influenced by both genetic and environmental factors,[48] a genetic disorder of polygenic/multifactorial inheritance[49] acquired via affected genes from either a person's father or mother. For example, children or siblings of women with endometriosis are at higher risk of developing endometriosis themselves; low progesterone levels may be genetic, and may contribute to a hormone imbalance.[50] Individuals with an affected first-degree relative have an approximate six-fold increase incidence of endometriosis.[51]
Inheritance is significant, but not the sole risk factor for endometriosis. Studies attribute 50% of risk to genetics, the other 50% likely to environmental factors.[52] It has been proposed that endometriosis may result from a series of multiple mutations, within target genes, in a mechanism similar to the development of cancer.[48] In this case, the mutations may be either somatic or heritable.[48]
A 2019 genome-wide association study (GWAS) review enumerated 36 genes with mutations associated with endometriosis development.[53] Nine chromosome loci were robustly replicated:[54][55][56][57]
| Chromosome | Gene/cytoband | Gene Product | Function |
|---|---|---|---|
| 1 | WNT4/1p36.12 | Wingless-type MMTV integration site family member 4 | Vital for development of the female reproductive organs |
| 2 | GREB1/2p25.1 | Growth regulation by estrogen in breast cancer 1/Fibronectin 1 | Early response gene in the estrogen regulation pathway/Cell adhesion and migration processes |
| 2 | ETAA1/2p14 | (ETAA1 Activator Of ATR Kinase) is a protein-coding gene. | Diseases associated with ETAA1 include Adult Lymphoma and Restless Legs Syndrome |
| 2 | IL1A/2q13 | Interleukin 1 alpha (IL-1α) is encoded by the IL1A gene. | Interleukin 1 alpha (IL-1α) is encoded by the IL1A gene. |
| 4 | KDR/4q12 | KDR is the human gene encoding kinase insert domain receptor also known as vascular endothelial growth factor receptor 2 (VEGFR-2) | Primary mediator of VEGF-induced endothelial proliferation, survival, migration, tubular morphogenesis and sprouting[58] |
| 6 | ID4/6p22.3 | Inhibitor of DNA binding 4 | Ovarian oncogene, biological function unknown |
| 7 | 7p15.2 | Transcription factors | Influence transcriptional regulation of uterine development |
| 9 | CDKN2BAS/9p21.3 | Cyclin-dependent kinase inhibitor 2B antisense RNA | Regulation of tumour suppressor genes |
| 12 | VEZT/12q22 | Vezatin, an adherens junction transmembrane protein | Tumor suppressor gene |
There are many findings of altered gene expression and epigenetics, but both of these can also be a secondary result of, for example, environmental factors and altered metabolism. Examples of altered gene expression include that of miRNAs.[48]
Environmental toxins
[edit]Some factors associated with endometriosis include:
- Prolonged exposure to naturally synthesized estrogen; for example, from late menopause[18] or early menarche[59][60]
- Obstruction of menstrual outflow; for example, in Müllerian anomalies[18]
Potential toxins:
- Dioxins- Several studies have investigated the potential link between exposure to dioxins and endometriosis, but evidence is equivocal and potential mechanisms are poorly understood.[61] A 2004 review of studies of dioxin and endometriosis concluded that "the human data supporting the dioxin-endometriosis association are scanty and conflicting",[62] and a 2009 follow-up review also found that there was "insufficient evidence" in support of a link between dioxin exposure and developing endometriosis.[63]
- Endocrine-disrupting chemicals (EDCs)- A wider class of hormonally active agents, to which dioxin belongs, consists of both natural and manmade compounds, e.g., bisphenols, phthalates, pesticides (chlorpyrifos, hexachlorobenzene) and polychlorinated biphenyls (PCBs).[64] Dietary uptake represents a significant source of EDC exposure via consumption of food, water and beverages, but exposure can also occur through ingestion of EDC dust and inhalation of its gases or particles in the air.[64] Most EDCs are lipophilic, allowing them to bioaccumulate in adipose tissue (body fat) and increase in concentration.[65] Bisphenol A (BPA), bisphenol S (BPS), phthalates, pesticides and PCBs all have a suspected linkage to endometriosis,[64] though have not been definitively proven as being causative.[65]
Vaginal dysbiosis
[edit]A growing body of evidence has shown a correlation between an imbalance in the vaginal microbiome and the appearance of endometriosis.[66] This correlation is mediated by an immune system overload in the context of retrograde menstruation, in which it fails to detect and kill cells that come outside of the vaginal environment. By disrupting normal immune function, dysbiosis leads to elevated levels of proinflammatory cytokines, a compromised immunosurveillance system and altered immune cell profiles. Indeed, the activation of Toll-like receptors in macrophages leads to a greater activity of this immune cell type. They, in turn, secrete factors (such as the pro-inflammatory cytokine interleukin 8) that help creating an inflammatory environment, ultimately favoring the proliferation and adhesion of endometrial cells.[66][67]
Pathophysiology
[edit]
While the exact cause of endometriosis remains unknown, many theories have been presented to better understand and explain its development. These concepts do not necessarily exclude each other. The pathophysiology of endometriosis is likely to be multifactorial and to involve an interplay between several factors.[48]
Formation
[edit]The main theories for the formation of the ectopic endometrium-like tissue include retrograde menstruation, Müllerianosis, coelomic metaplasia, vascular dissemination of stem cells, and surgical transplantation were postulated as early as 1870. Each is further described below.[14][68][69]
Retrograde menstruation theory
[edit]The theory of retrograde menstruation (also called the implantation theory or transplantation theory) is the most commonly accepted theory for the dissemination and transformation of ectopic endometrium into endometriosis. It suggests that during a woman's menstrual flow, some of the endometrial debris flow backward through the fallopian tubes and into the peritoneal cavity, attaching itself to the peritoneal surface (the lining of the abdominal cavity) where it can proceed to invade the tissue as or transform into endometriosis. It is not clear at what stage the transformation of endometrium, or any cell of origin such as stem cells or coelomic cells (see those theories below), to endometriosis begins.[48][68][70]
Proofs in support of the theory are based on retrospective epidemiological studies that an association with endometrial implants attached to the peritoneal cavity, which would develop into endometrial lesions and retrograde menstruation; and the fact that animals like rodents and non-human primates whose endometrium is not shed during the estrous cycle don't develop naturally endometriosis contrary to animals that have a natural menstrual cycle like rhesus monkeys and baboons.[71]
Retrograde menstruation alone is not able to explain all instances of endometriosis, and additional factors such as genetics, immunology, stem cell migration, and coelomic metaplasia (see "Other theories" on this page) are needed to account for disseminated disease and why many individuals with retrograde menstruation are not diagnosed with endometriosis. In addition, endometriosis has shown up in people who have never experienced menstruation including cisgender men,[72] fetuses,[73] and prepubescent girls.[74][75] Further theoretical additions are needed to complement the retrograde menstruation theory to explain why cases of endometriosis show up in the brain[76] and lungs.[77]
Researchers are investigating the possibility that the immune system may not be able to cope with the cyclic onslaught of retrograde menstrual fluid. In this context there is interest in studying the relationship of endometriosis to autoimmune disease, allergic reactions, and the impact of toxic materials.[17][78] It is still unclear what, if any, causal relationship exists between toxic materials or autoimmune disease and endometriosis. There are immune system changes in people with endometriosis, such as an increase of macrophage-derived secretion products, but it is unknown if these are contributing to the disorder or are reactions from it.[79]
Endometriotic lesions differ in their biochemistry, hormonal response, immunology, inflammatory response when compared to endometrium.[14][80] This is likely because the cells that give rise to endometriosis are a side population of cells.[48] Similarly, there are changes in, for example, the mesothelium of the peritoneum in people with endometriosis, such as loss of tight junctions, but it is unknown if these are causes or effects of the disorder.[79]
In rare cases where imperforate hymen does not resolve itself prior to the first menstrual cycle and goes undetected, blood and endometrium are trapped within the uterus until such time as the problem is resolved by surgical incision. Many health care practitioners never encounter this defect, and due to the flu-like symptoms it is often misdiagnosed or overlooked until multiple menstrual cycles have passed. By the time a correct diagnosis has been made, endometrium and other fluids have filled the uterus and Fallopian tubes with results similar to retrograde menstruation resulting in endometriosis. The initial stage of endometriosis may vary based on the time elapsed between onset and surgical procedure.[citation needed]
The theory of retrograde menstruation as a cause of endometriosis was first proposed by John A. Sampson.[68][81]
Other theories
[edit]- Stem cells: Endometriosis may arise from stem cells from bone marrow and potentially other sources. In particular, this theory explains endometriosis found in areas remote from the pelvis such as the brain or lungs.[69] Stem cells may be from local cells such as the peritoneum (see coelomic metaplasia below) or cells disseminated in the blood stream (see vascular dissemination below) such as those from the bone marrow.[68][69][82]
- Vascular dissemination: Vascular dissemination is a 1927 theory that has been revived with new studies of bone-marrow stem cells involved in pathogenesis.[69][82]
- Environment: Environmental toxins (e.g., dioxin, nickel) may cause endometriosis.[83][84] Toxins such as dioxins and dioxin-like compounds tend to bioaccumulate within the human body. Further research is needed but "it is plausible that inflammatory-like processes, caused by dioxin-like environmental chemicals, can alter normal endometrial and immune cell physiology allowing persistence and development of endometrial tissue within the peritoneal cavity, normally cleared by immune system cells".[85]
- Müllerianosis: A theory supported by foetal autopsy is that cells with the potential to become endometrial, which are laid down in tracts during embryonic development called the female reproductive (Müllerian) tract as it migrates downward at 8–10 weeks of embryonic life, could become dislocated from the migrating uterus and act like seeds or stem cells.[68][73]
- Coelomic metaplasia: Coelomic cells which are the common ancestor of endometrial and peritoneal cells may undergo metaplasia (transformation) from one type of cell to the other, perhaps triggered by inflammation.[68][86]
- Vasculogenesis: Up to 37% of the microvascular endothelium of ectopic endometrial tissue originates from endothelial progenitor cells, which result in de novo formation of microvessels by the process of vasculogenesis rather than the conventional process of angiogenesis.[87][clarification needed]
- Neural growth: An increased expression of new nerve fibres is found in endometriosis but does not fully explain the formation of ectopic endometriotic tissue and is not definitely correlated with the amount of perceived pain.[88][clarification needed]
- Autoimmune: Graves disease is an autoimmune disease characterized by hyperthyroidism, goiter, ophthalmopathy, and dermopathy. People with endometriosis had higher rates of Graves disease. One of these potential links between Graves disease and endometriosis is autoimmunity.[89][90]
- Oxidative stress: Influx of iron is associated with the local destruction of the peritoneal mesothelium, leading to the adhesion of ectopic endometriotic cells.[91] Peritoneal iron overload has been suggested to be caused by the destruction of erythrocytes, which contain the iron-binding protein hemoglobin, or a deficiency in the peritoneal iron metabolism system.[91] Oxidative stress activity and reactive oxygen species (ROS) (such as superoxide anions and peroxide levels) are reported to be higher than normal in people with endometriosis.[91] Oxidative stress and the presence of excess ROS can damage tissue and induce rapid cellular division.[91] Mechanistically, there are several cellular pathways by which oxidative stress may lead to or may induce proliferation of endometriotic lesions, including the mitogen activated protein (MAP) kinase pathway and the extracellular signal-related kinase (ERK) pathway.[91] Activation of both of the MAP and ERK pathways lead to increased levels of c-Fos and c-Jun, which are proto-oncogenes that are associated with high-grade lesions.[91]
Localization
[edit]Most often, endometriosis is found on the:
- Ovaries
- Fallopian tubes
- Tissues that hold the uterus in place (ligaments)
- Outer surface of the uterus[3]
Less common pelvic sites are:
Endometriosis may spread to the cervix and vagina or to sites of a surgical abdominal incision, known as "scar endometriosis."[92] Rectovaginal or bowel endometriosis affects approximately 5-12% of those with endometriosis, and can cause severe pain with bowel movements.[93][citation needed]
Deep infiltrating endometriosis (DIE) has been defined as the presence of endometrial glands and stroma infiltrating more than 5 mm in the subperitoneal tissue. The prevalence of DIE is estimated to be 1 to 2% in women of reproductive age. Deep endometriosis typically presents as a single nodule in the vesicouterine fold or in the lower 20 cm of the bowel. Deep endometriosis can be associated with severe pain. However, it can be present without severe levels of pain.[94]
Male endometriosis
[edit]Endometriosis has been reported in people assigned male at birth. Prostate endometriosis has been reported following estrogen therapy for prostate cancer[95] and feminizing hormone therapy.[96]
Abdominal endometriosis also happens in men following cirrhosis.[97]
Extrapelvic endometriosis
[edit]Rarely, endometriosis appears in extrapelvic parts of the body, such as the lungs, brain, and skin.[3][39][92] "Scar endometriosis" can occur in surgical abdominal incisions.[92] Risk factors for scar endometriosis include previous abdominal surgeries, such as a hysterotomy or cesarean section, or ectopic pregnancies, salpingostomy puerperal sterilization, laparoscopy, amniocentesis, appendectomy, episiotomy, vaginal hysterectomies, and hernia repair.[98][99][100]
Endometriosis may also present with skin lesions in cutaneous endometriosis.[92]
Less commonly lesions can be found on the diaphragm or lungs. Diaphragmatic endometriosis is rare, almost always on the right hemidiaphragm, and may inflict the cyclic pain of the right scapula (shoulder) or cervical area (neck) during a menstrual period.[101] Pulmonary endometriosis can be associated with a thoracic endometriosis syndrome that can include catamenial (occurs during menstruation) pneumothorax seen in 73% of women with the syndrome, catamenial hemothorax in 14%, catamenial hemoptysis in 7%, and pulmonary nodules in 6%.[39]
Diagnosis
[edit]
A health history and a physical examination can lead the health care practitioner to suspect endometriosis. There is a clear benefit for performing a transvaginal ultrasound (TVUS) as a first step of testing for endometriosis.[94]
Definitive diagnosis is based on the morphology (form and structure) of the pelvic region, determined by observation (surgical or non-invasive imaging), classified into four different stages of endometriosis. The American Society of Reproductive Medicine's scale, revised in 1996, gives higher scores to deep, thick lesions or intrusions on the ovaries and dense, enveloping adhesions on the ovaries or fallopian tubes.[102] Additionally, histological studies, when performed, should show specific findings.
For many patients, there are significant delays in diagnosis. Studies show an average delay of 11.7 years in the United States. Patients in the UK have an average delay of 8 years and in Norway of 6.7 years.[103] A third of women had consulted their GP six or more times before being diagnosed.[103]
The most common sites of endometriosis are the ovaries, followed by the Douglas pouch, the posterior leaves of the broad ligaments, and the sacrouterine ligaments.[22]
As for deep infiltrating endometriosis, TVUS, TRUS and MRI are the techniques of choice for non-invasive diagnosis with a high sensitivity and specificity.[104]
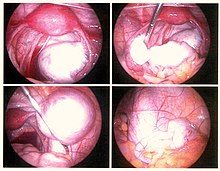
Laparoscopy
[edit]
Laparoscopy, a surgical procedure where a camera is used to look inside the abdominal cavity, is the only way to accurately diagnose the extent and severity of pelvic/abdominal endometriosis.[105] Laparoscopy is not an applicable test for extrapelvic sites such as umbilicus, hernia sacs, abdominal wall, lung, or kidneys.[105]
Reviews in 2019 and 2020 concluded that 1) with advances in imaging, endometriosis diagnosis should no longer be considered synonymous with immediate laparoscopy for diagnosis, and 2) endometriosis should be classified a syndrome that requires confirmation of visible lesions seen at laparoscopy in addition to characteristic symptoms.[106][107]
Laparoscopy permits lesion visualization unless the lesion is visible externally (e.g., an endometriotic nodule in the vagina) or is extra-abdominal.[105] If the growths (lesions) are not visible, a biopsy must be taken to determine the diagnosis.[108] Surgery for diagnoses also allows for surgical treatment of endometriosis at the same time.
During a laparoscopic procedure, lesions can appear dark blue, powder-burn black, red, white, yellow, brown or non-pigmented. Lesions vary in size.[109] Some within the pelvis walls may not be visible, as normal-appearing peritoneum of infertile women reveals endometriosis on biopsy in 6–13% of cases.[110] Early endometriosis typically occurs on the surfaces of organs in the pelvic and intra-abdominal areas.[109] Health care providers may call areas of endometriosis by different names, such as implants, lesions, or nodules. Larger lesions may be seen within the ovaries as endometriomas or "chocolate cysts", "chocolate" because they contain a thick brownish fluid, mostly old blood.[109]
Frequently during diagnostic laparoscopy, no lesions are found in individuals with chronic pelvic pain, a symptom common to other disorders including adenomyosis, pelvic adhesions, pelvic inflammatory disease, congenital anomalies of the reproductive tract, and ovarian or tubal masses.[111]
Ultrasound
[edit]Vaginal ultrasound can be used to diagnosis endometriosis, or for localizing endometrioma before surgery.[112] This can be used to identify the spread of disease in individuals with well-established clinical suspicion of endometriosis.[112] Vaginal ultrasound is inexpensive, easily accessible, has no contraindications and requires no preparation.[112] By extending the ultrasound assessment into the posterior and anterior pelvic compartments a sonographer is able to evaluate structural mobility and look for deep infiltrating endometriotic nodules.[113] Better sonographic detection of deep infiltrating endometriosis could reduce the number of diagnostic laparoscopies, as well as guide disease management and enhance patient quality of life.[113]
Magnetic resonance imaging
[edit]MRI is another means of detecting lesions in a non-invasive manner.[105] MRI is not widely used due to its cost and limited availability, although it can be used to detect the most common form of endometriosis (endometrioma) with a sufficient accuracy.[105] A 2020 article recommended administering an anti-spasmodic agent (i.e. hyoscine butylbromide) and a big glass of water (if the bladder is empty), and scanning in the supine position with an abdominal strap, for better image quality.[114] It also recommended using pelvic-phased array coils and T1 (spin-lattice) weighted scanning, with and without suppression of fat for endometriomas, and sagittal, axial and oblique 2D T2 (spin-spin) weighting for deep infiltrating endometriosis.[114]
Stages of disease
[edit]By surgical observation, endometriosis can be classified as stage I–IV by the 1996 scale of the American Society of Reproductive Medicine (ASRM).[102] The scale uses a point system that assesses lesions and adhesions in the pelvic organs. It is important to note that staging assesses physical disease only, not the level of pain or infertility.[115] A person with Stage I endometriosis may have a little disease and severe pain, while a person with Stage IV endometriosis may have severe disease and no pain or vice versa. The various stages are summarized by:
Stage I (Minimal)
- Findings restricted to only superficial lesions and possibly a few filmy adhesions.
Stage II (Mild)
- In addition, some deep lesions are present in the cul-de-sac.
Stage III (Moderate)
- As above, plus the presence of endometriomas on the ovary and more adhesions.
Stage IV (Severe)
- As above, plus large endometriomas, extensive adhesions. Implants and adhesions may be found beyond the uterus. Large ovarian cysts are common.
Markers
[edit]An area of research is the search for endometriosis markers.[116]
In 2010, essentially all proposed biomarkers for endometriosis were of unclear medical use, although some appear to be promising.[116] The one biomarker that has been in use over the last 20 years is CA-125.[116] A 2016 review found that this biomarker was present in those with symptoms of endometriosis; and, once ovarian cancer has been ruled out, a positive CA-125 may confirm the diagnosis.[117] Its performance in ruling out endometriosis is low.[117] CA-125 levels appear to fall during endometriosis treatment, but it has not shown a correlation with disease response.[116]
Another review in 2011 identified several putative biomarkers upon biopsy, including findings of small sensory nerve fibers or defectively expressed β3 integrin subunit.[118] It has been postulated a future diagnostic tool for endometriosis will consist of a panel of several specific and sensitive biomarkers, including both substance concentrations and genetic predisposition.[116]
A 2016 review of endometrial biomarkers for diagnosing endometriosis was unable to draw conclusions due to the low quality of the evidence.[119]
MicroRNAs have the potential to be used in diagnostic and therapeutic decisions.[120]
Histopathology
[edit]For a histopathological diagnosis, at least two of the following three criteria should be present:[121]
- Endometrial type stroma
- Endometrial epithelium with glands
- Evidence of chronic hemorrhage, mainly hemosiderin deposits
Immunohistochemistry has been found to be useful in diagnosing endometriosis as stromal cells have a peculiar surface antigen, CD10, thus allowing the pathologist go straight to a staining area and confirm the presence of stromal cells and sometimes glandular tissue is identified that was missed on routine H&E staining.[122]
-
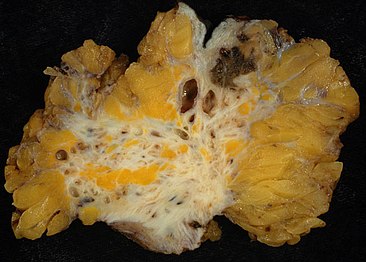
Endometriosis, abdominal wall
-
Micrograph showing endometriosis (right) and ovarian stroma (left)
-
Micrograph of the wall of an endometrioma. All features of endometriosis are present (endometrial glands, endometrial stroma and hemosiderin-laden macrophages).
Pain quantification
[edit]The most common pain scale for quantification of endometriosis-related pain is the visual analogue scale (VAS); VAS and numerical rating scale (NRS) were the best adapted pain scales for pain measurement in endometriosis. For research purposes, and for more detailed pain measurement in clinical practice, VAS or NRS for each type of typical pain related to endometriosis (dysmenorrhea, deep dyspareunia and non-menstrual chronic pelvic pain), combined with the clinical global impression (CGI) and a quality of life scale, are used.[123]
Prevention
[edit]Limited evidence indicates that the use of combined oral contraceptives is associated with a reduced risk of endometriosis, as is regular exercise and the avoidance of alcohol and caffeine.[3] There is little known information on preventing endometriosis.[124]
Management
[edit]While there is no cure for endometriosis, there are two types of interventions; treatment of pain and treatment of endometriosis-associated infertility.[125] In many cases, menopause (natural or surgical) will abate the process.[126] In the reproductive years, endometriosis is merely managed: the goal is to provide pain relief, to restrict progression of the process, and to restore or preserve fertility where needed. In younger individuals, some surgical treatment attempts to remove endometriotic tissue and preserve the ovaries without damaging normal tissue.[14][127]
Pharmacotherapy for pain management can be initiated based on the presence of symptoms and examination and ultrasound findings that rule out other potential causes.[128]
In general, the diagnosis of endometriosis is confirmed during surgery, at which time removal can be performed. Further steps depend on circumstances: someone without infertility can manage symptoms with pain medication and hormonal medication that suppresses the natural cycle, while an infertile individual may be treated expectantly after surgery, with fertility medication, or with in vitro fertilisation (IVF).
A 2020 Cochrane systematic review found that for all types of endometriosis, "it is uncertain whether laparoscopic surgery improves overall pain compared to diagnostic laparoscopy".[129]
Surgery
[edit]Based on strong evidence, experts recommend that surgery be performed laparoscopically (through keyhole surgery) rather than open.[108] Treatment consists of the ablation or excision of the endometriosis, electrocoagulation,[130] lysis of adhesions, resection of endometriomas, and restoration of normal pelvic anatomy as much as is possible.[108][131] When laparoscopic surgery is used, small instruments are inserted through the incisions to remove the endometriosis tissue and adhesions. Because the incisions are very small, there will only be small scars on the skin after the procedure, and most individuals recover from surgery quickly and have a reduced risk of adhesions.[132] Many endometriosis specialists believe that excision is the ideal surgical method to treat endometriosis.[133] A 2017 literature review found excision improved some outcomes over ablation.[134] In the United States, some specialists trained in excision for endometriosis do not accept health insurance, because insurance companies do not reimburse the higher costs of this procedure over ablation.[135]
As for deep endometriosis, a segmental resection or shaving of nodules is effective but is associated with an increased rate of complications, of which about 4.6% are major.[136]
Historically, a hysterectomy (removal of the uterus) was thought to be a cure for endometriosis in individuals who do not wish to conceive. Removal of the uterus may be beneficial as part of the treatment, if the uterus itself is affected by adenomyosis. However, this should only be done in combination with removal of the endometriosis by excision. If endometriosis is not also removed at the time of hysterectomy, pain may persist.[108] A study of hysterectomy patients found those with endometriosis were not using less pain medication 3 years after the procedure.[137]
Presacral neurectomy may be performed where the nerves to the uterus are cut. However, this technique is not usually used due to the high incidence of associated complications including presacral hematoma and irreversible problems with urination and constipation.[108]
Recurrence
[edit]The underlying process that causes endometriosis may not cease after a surgical or medical intervention. A study has shown that dysmenorrhea recurs at a rate of 30 percent within a year following laparoscopic surgery. Resurgence of lesions tend to appear in the same location if the lesions were not completely removed during surgery. It has been shown that laser ablation resulted in higher and earlier recurrence rates when compared with endometrioma cystectomy; and recurrence after repetitive laparoscopy was similar to that after the first surgery. Endometriosis has a 10% recurrence rate after hysterectomy and bilateral salpingo-oophorectomy.[138]
Endometriosis recurrence following conservative surgery is estimated as 21.5% at 2 years and 40-50% at 5 years.[139]
Recurrence rate for DIE after surgery is less than 1%.[140]
Risks and safety of pelvic surgery
[edit]Risk of developing complications following surgery depend on the type of the lesion that has undergone surgery.[130] 55% to 100% of individuals develop adhesions following pelvic surgery,[141] which can result in infertility, chronic abdominal and pelvic pain, and difficult reoperative surgery. Trehan's temporary ovarian suspension, a technique in which the ovaries are suspended for a week after surgery, may be used to reduce the incidence of adhesions after endometriosis surgery.[142][143] Removal of cysts on the ovary without removing the ovary is a safe procedure.[130]
Hormonal medications
[edit]- Hormonal birth control therapy: Birth control pills reduce the menstrual pain and recurrence rate for endometrioma following conservative surgery for endometriosis.[144] A 2018 Cochrane systematic review found that there is insufficient evidence to make a judgement on the effectiveness of the combined oral contraceptive pill compared with placebo or other medical treatment for managing pain associated with endometriosis partly because of lack of included studies for data analysis (only two for COCP vs placebo).[145]
- Progestogens: Progesterone counteracts estrogen and inhibits the growth of the endometrium.[146] Danazol and gestrinone are suppressive steroids with some androgenic activity.[127] Both agents inhibit the growth of endometriosis but their use has declined, due in part to virilizing side effects such as excessive hair growth and voice changes.[147] There is tentative evidence based on cohort studies that dienogest and norethisterone acetate (NETA) may help patients with DIE in terms of pain.[148] There is tentative evidence based on a prospective study that vaginal danazol reduces pain in those affected by DIE.[148]
- Gonadotropin-releasing hormone (GnRH) modulators: These drugs include GnRH agonists such as leuprorelin and GnRH antagonists such as elagolix and are thought to work by decreasing estrogen levels.[149] A 2010 Cochrane review found that GnRH modulators were more effective for pain relief in endometriosis than no treatment or placebo, but were not more effective than danazol or intrauterine progestogen, and had more side effects than danazol.[149] A 2018 Swedish systematic review found that GnRH modulators had similar pain-relieving effects to gestagen, but also decreased bone density.[112]
- Aromatase inhibitors are medications that block the formation of estrogen and have become of interest for researchers who are treating endometriosis.[150] Examples of aromatase inhibitors include anastrozole and letrozole. Evidence for aromatase inhibitors is confirmed by numerous controlled studies that show benefit in terms of pain control and quality of life when used in combination with gestagens or oral contraceptives with less side-effects when used in combination with oral contraceptives like norethisterone acetate.[151] Despite multiple benefits, there are lot of things to consider before using aromatase inhibitors for endometriosis, as it is common for them to induce functional cysts as an adverse effects. Moreover, dosages, treatment length, appropriate add-back therapies and mode of administration is still being investigated.[152]
- Progesterone receptor modulators like mifepristone and gestrinone have the potential (based on only one randomized controlled trial each) to be used as a treatment to manage pain caused by endometriosis.[153]
Other medicines
[edit]- Melatonin, there is tentative evidence for its use (at a dose of 10 mg) in reducing pain related to endometriosis.[154]
- Vitamin C and E antioxidant supplementation may significantly reduce pain symptoms in endometriosis.[155]
- Opioids: Morphine sulphate tablets and other opioid painkillers work by mimicking the action of naturally occurring pain-reducing chemicals called "endorphins". There are different long acting and short acting medications that can be used alone or in combination to provide appropriate pain control.
- Chinese herbal medicine was reported to have comparable benefits to gestrinone and danazol in patients who had had laparoscopic surgery, though the review notes that the two trials were small and of "poor methodological quality" and results should be "interpreted cautiously" as better quality research is needed.[156]
- Serrapeptase, a digestive enzyme found in the intestines of silkworms. Serrapeptase is widely used in Japan and Europe as an anti-inflammatory treatment.[157] More research is needed but serrapeptase may be used by endometriosis patients to reduce inflammation.[158]
- Angiogenesis inhibitors lack clinical evidence of efficacy in endometriosis therapy.[159] Under experimental in vitro and in vivo conditions, compounds that have been shown to exert inhibitory effects on endometriotic lesions include growth factor inhibitors, endogenous angiogenesis inhibitors, fumagillin analogues, statins, cyclo-oxygenase-2 inhibitors, phytochemical compounds, immunomodulators, dopamine agonists, peroxisome proliferator-activated receptor agonists, progestins, danazol and gonadotropin-releasing hormone agonists.[159] However, many of these agents are associated with undesirable side effects and more research is necessary. An ideal therapy would diminish inflammation and underlying symptoms without being contraceptive.[160][161]
- Pentoxifylline, an immunomodulating agent, has been theorized to improve pain as well as improve pregnancy rates in individuals with endometriosis. There is not enough evidence to support the effectiveness or safety of either of these uses.[162] Current American Congress of Obstetricians and Gynecologists (ACOG) guidelines do not include immunomodulators, such as pentoxifylline, in standard treatment protocols.[163]
- NSAIDs are anti-inflammatory medications commonly used for endometriosis patients despite unproven efficacy and unintended adverse effects.[164]
- Neuromodulators like gabapentin did not prove to be superior to placebo in managing pain caused by endometriosis.[165]
The overall effectiveness of manual physical therapy to treat endometriosis has not yet been identified.[166]
Comparison of interventions
[edit]A 2021 meta-analysis found that GnRH analogues and combined hormonal contraceptives were the best treatment for reducing dyspareunia, menstrual and non menstrual pelvic pain.[167] A 2018 Swedish systematic review found a large number of studies but a general lack of scientific evidence for most treatments.[112] There was only one study of sufficient quality and relevance comparing the effect of surgery and non-surgery.[168] Cohort studies indicate that surgery is effective in decreasing pain.[168] Most complications occurred in cases of low intestinal anastomosis, while risk of fistula occurred in cases of combined abdominal or vaginal surgery, and urinary tract problems were common in intestinal surgery.[168] The evidence was found to be insufficient regarding surgical intervention.[168]
The advantages of physical therapy techniques are decreased cost, absence of major side-effects, it does not interfere with fertility, and near-universal increase of sexual function.[169] Disadvantages are that there are no large or long-term studies of its use for treating pain or infertility related to endometriosis.[169]
Treatment of infertility
[edit]Surgery is more effective than medicinal intervention for addressing infertility associated with endometriosis.[127] Surgery attempts to remove endometrium-like tissue[14] and preserve the ovaries without damaging normal tissue.[127] Receiving hormonal suppression therapy after surgery might be positive regarding endometriosis recurrence and pregnancy.[170] In-vitro fertilization (IVF) procedures are effective in improving fertility in many individuals with endometriosis.[1]
During fertility treatment, the ultralong pretreatment with GnRH-agonist has a higher chance of resulting in pregnancy for individuals with endometriosis, compared to the short pretreatment.[112]
Research
[edit]Preliminary research on mouse models showed that monoclonal antibodies, as well as inhibitors of MyD88 downstream signaling pathway, can reduce lesion volume. Thanks to that, clinical trials are being done on using a monoclonal antibody directed against IL-33 and using anakinra, an IL-1 receptor antagonist.[165]
Promising preclinical outcomes is pushing clinical trials into testing cannabinoid extracts, dichloroacetic acid and curcuma capsules.[165]
Epidemiology
[edit]Determining how many people have endometriosis is challenging because definitive diagnosis requires surgical visualization through laparoscopic surgery.[171] Criteria that are commonly used to establish a diagnosis include pelvic pain, infertility, surgical assessment, and in some cases, magnetic resonance imaging. An ultrasound can identify large clumps of tissue as potential endometriosis lesions and ovarian cysts but it is not effective for all patients, especially in cases with smaller, superficial lesions.[172]
Ethnic differences in endometriosis have been observed. The condition is more common in women of East Asian and Southeast Asian descent than in White women.[14] Risk factors include having a family history of the condition.[15]
One estimate is that 10.8 million people are affected globally as of 2015[update].[6] Other sources estimate 6 to 10% of the general female population[1] and 2 to 11% of asymptomatic women[14] are affected. In addition, 11% of women in a general population have undiagnosed endometriosis that can be seen on magnetic resonance imaging (MRI).[173][171] Endometriosis is most common in those in their thirties and forties; however, it can begin in girls as early as eight years old.[3][4] It results in few deaths with unadjusted and age-standardized death rates of 0.1 and 0.0 per 100,000.[6] Endometriosis was first determined to be a separate condition in the 1920s.[174] Before that time, endometriosis and adenomyosis were considered together.[174] It is unclear who first described the disease.
It chiefly affects adults from premenarche to postmenopause, regardless of race or ethnicity or whether or not they have had children and is estimated to affect over 190 million women in their reproductive years.[175] Incidences of endometriosis have occurred in postmenopausal individuals,[176] and in less common cases, individuals may have had endometriosis symptoms before they even reach menarche.[177][75]
The rate of recurrence of endometriosis is estimated to be 40-50% for adults over a 5-year period.[178] The rate of recurrence has been shown to increase with time from surgery and is not associated with the stage of the disease, initial site, surgical method used, or post-surgical treatment.[178]
History
[edit]Endometriosis was first discovered microscopically by Karl von Rokitansky in 1860,[179] although the earliest antecedents may have stemmed from concepts published almost 4,000 years ago.[180] The Hippocratic Corpus outlines symptoms similar to endometriosis, including uterine ulcers, adhesions, and infertility.[180] Historically, women with these symptoms were treated with leeches, straitjackets, bloodletting, chemical douches, genital mutilation, pregnancy (as a form of treatment), hanging upside down, surgical intervention, and even killing due to suspicion of demonic possession.[180] Hippocratic doctors recognized and treated chronic pelvic pain as a true organic disorder 2,500 years ago, but during the Middle Ages, there was a shift into believing that women with pelvic pain were mad, immoral, imagining the pain, or simply misbehaving.[180] The symptoms of inexplicable chronic pelvic pain were often attributed to imagined madness, female weakness, promiscuity, or hysteria.[180] The historical diagnosis of hysteria, which was thought to be a psychological disease, may have indeed been endometriosis.[180] The idea that chronic pelvic pain was related to mental illness influenced modern attitudes regarding individuals with endometriosis, leading to delays in correct diagnosis and indifference to the patients' true pain throughout the 20th and into the 21st century.[180]
Hippocratic doctors believed that delaying childbearing could trigger diseases of the uterus, which caused endometriosis-like symptoms. Women with dysmenorrhea were encouraged to marry and have children at a young age.[180] The fact that Hippocratics were recommending changes in marriage practices due to an endometriosis-like illness implies that this disease was likely common, with rates higher than the 5-15% prevalence that is often cited today.[180] If indeed this disorder was so common historically, this may point away from modern theories that suggest links between endometriosis and dioxins, PCBs, and chemicals.[180]
The early treatment of endometriosis was surgical and included oophorectomy (removal of the ovaries) and hysterectomy (removal of the uterus).[181] In the 1940s, the only available hormonal therapies for endometriosis were high-dose testosterone and high-dose estrogen therapy.[182] High-dose estrogen therapy with diethylstilbestrol for endometriosis was first reported by Karnaky in 1948 and was the main pharmacological treatment for the condition in the early 1950s.[183][184][185] Pseudopregnancy (high-dose estrogen–progestogen therapy) for endometriosis was first described by Kistner in the late 1950s.[183][184] Pseudopregnancy as well as progestogen monotherapy dominated the treatment of endometriosis in the 1960s and 1970s.[185] These agents, although efficacious, were associated with intolerable side effects. Danazol was first described for endometriosis in 1971 and became the main therapy in the 1970s and 1980s.[183][184][185] In the 1980s GnRH agonists gained prominence for the treatment of endometriosis and by the 1990s had become the most widely used therapy.[184][185] Oral GnRH antagonists such as elagolix were introduced for the treatment of endometriosis in 2018.[186]
Society and culture
[edit]Public figures
[edit]A number of public figures have spoken about their experience with endometriosis, including:
- RuthAnne[187]
- Emma Barnett[188]
- Emma Bunton[189]
- Alexa Chung[190]
- Danielle Collins[191]
- Olivia Culpo[192]
- Lena Dunham[193]
- Abby Finkenauer[194]
- Bethenny Frankel[195]
- Whoopi Goldberg[196]
- Mel Greig[197]
- Halsey[198]
- Emma Hayes[199]
- Julianne Hough[200][201][202]
- Bridget Hustwaite[203]
- Bindi Irwin[204]
- Jaime King[205]
- Padma Lakshmi[206]
- Cyndi Lauper[207]
- Jillian Michaels[208]
- Monica[209]
- Marilyn Monroe[210]
- Tia Mowry[211]
- Sinéad O'Connor[212]
- Dolly Parton[213]
- Daisy Ridley[214]
- Emma Roberts[215]
- Susan Sarandon[216]
- Amy Schumer[217]
- Kirsten Storms[218]
- Gabrielle Union[219]
- Lacey Schwimmer[220][221][222]
- Chrissy Teigen[223]
- Emma Watkins[224]
- Mae Whitman[225]
- Jessica Williams[226]
- Leah Williamson[227]
Economic burden
[edit]The economic burden of endometriosis is widespread and multifaceted.[228] Endometriosis is a chronic disease that has direct and indirect costs which include loss of work days, direct costs of treatment, symptom management, and treatment of other associated conditions such as depression or chronic pain.[228] One factor which seems to be associated with especially high costs is the delay between onset of symptoms and diagnosis.
Costs vary greatly between countries.[229] Two factors that contribute to the economic burden include healthcare costs and losses in productivity. A Swedish study of 400 endometriosis patients found "Absence from work was reported by 32% of the women, while 36% reported reduced time at work because of endometriosis".[230] An additional cross sectional study with Puerto Rican women, "found that endometriosis-related and coexisting symptoms disrupted all aspects of women's daily lives, including physical limitations that affected doing household chores and paid employment. The majority of women (85%) experienced a decrease in the quality of their work; 20% reported being unable to work because of pain, and over two-thirds of the sample continued to work despite their pain."[231]
Medical culture
[edit]There are a number of barriers that those affected face to receiving diagnosis and treatment for endometriosis. Some of these include outdated standards for laparoscopic evaluation, stigma about discussing menstruation and sex, lack of understanding of the disease, primary-care physicians' lack of knowledge, and assumptions about typical menstrual pain.[232] On average, those later diagnosed with endometriosis waited 2.3 years after the onset of symptoms before seeking treatment and nearly three quarters of women receive a misdiagnosis prior to endometriosis.[233] Self-help groups say practitioners delay making the diagnosis, often because they do not consider it a possibility. There is a typical delay of 7–12 years from symptom onset in affected individuals to professional diagnosis.[234] There is a general lack of knowledge about endometriosis among primary care physicians. Half of general health care providers surveyed in a 2013 study were unable to name three symptoms of endometriosis.[235] Health care providers are also likely to dismiss described symptoms as normal menstruation.[236] Younger patients may also feel uncomfortable discussing symptoms with a physician.[236]
Race and ethnicity
[edit]Race and ethnicity may play a role in how endometriosis affects one's life. Endometriosis is less thoroughly studied among Black people, and the research that has been done is outdated.[237] [238] Cultural differences among ethnic groups also contribute to attitudes toward and treatment of endometriosis, especially in Hispanic or Latino communities. A study done in Puerto Rico in 2020 found that health care and interactions with friends and family related to discussing endometriosis were affected by stigma.[239] The most common finding was a referral to those expressing pain related to endometriosis as "changuería" or "changas", terms used in Puerto Rico to describe pointless whining and complaining, often directed at children.[239]
Stigma
[edit]The existing stigma surrounding women's health, specifically endometriosis, can lead to patients not seeking diagnoses, lower quality of healthcare, increased barriers to care and treatment, and negative reception from members of society.[240] Additionally, menstrual stigma significantly contributes to the broader issue of endometriosis stigma, creating an interconnected challenge that extends beyond reproductive health.[241][242] Widespread awareness campaigns, developments and implementations aimed to multilevel anti-stigma organizational and structural changes, as well as more qualitative studies of the endometriosis stigma, help to overcome the harm of the phenomenon.[243]
References
[edit]- ^ a b c d e f g h i j k l m n o p Bulletti C, Coccia ME, Battistoni S, Borini A (August 2010). "Endometriosis and infertility". Journal of Assisted Reproduction and Genetics. 27 (8): 441–7. doi:10.1007/s10815-010-9436-1. PMC 2941592. PMID 20574791.
- ^ Horne AW, Missmer SA (November 2022). "Pathophysiology, diagnosis, and management of endometriosis". BMJ. 379: e070750. doi:10.1136/bmj-2022-070750. hdl:20.500.11820/a2c07717-cf08-4119-b0f4-b7f8aa50193e. PMID 36375827.
- ^ a b c d e f g h i j k l m n o p q r s "Endometriosis". Office on Women's Health. 13 February 2017. Archived from the original on 13 May 2017. Retrieved 20 May 2017.
- ^ a b McGrath PJ, Stevens BJ, Walker SM, Zempsky WT (2013). Oxford Textbook of Paediatric Pain. OUP Oxford. p. 300. ISBN 978-0-19-964265-6. Archived from the original on 10 September 2017.
- ^ Parasar P, Ozcan P, Terry KL (2017). "Endometriosis: Epidemiology, Diagnosis and Clinical Management". Curr Obstet Gynecol Rep. 6 (1): 34–41. doi:10.1007/s13669-017-0187-1. PMC 5737931. PMID 29276652.
- ^ a b c d e Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ^ Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. (GBD 2015 Mortality and Causes of Death Collaborators) (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ^ "Endometriosis: Overview". nichd.nih.gov. Archived from the original on 18 May 2017. Retrieved 20 May 2017.
- ^ "Endometriosis: Condition Information". nichd.nih.gov. Archived from the original on 30 April 2017. Retrieved 20 May 2017.
- ^ "Endometriosis Is More Than Just 'Painful Periods'". Yale Medicine. Retrieved 12 October 2023.
- ^ Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ (April 1991). "Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain". Fertility and Sterility. 55 (4): 759–65. doi:10.1016/s0015-0282(16)54244-7. PMID 2010001. S2CID 29998004.
- ^ a b Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, et al. (1 November 2013). "The social and psychological impact of endometriosis on women's lives: a critical narrative review". Human Reproduction Update. 19 (6): 625–39. doi:10.1093/humupd/dmt027. hdl:2086/8845. PMID 23884896.
- ^ Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C, et al. (August 2011). "Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries". Fertility and Sterility. 96 (2): 366–373.e8. doi:10.1016/j.fertnstert.2011.05.090. PMC 3679489. PMID 21718982.
- ^ a b c d e f g h Zondervan KT, Becker CM, Missmer SA (March 2020). "Endometriosis". The New England Journal of Medicine. 382 (13): 1244–1256. doi:10.1056/NEJMra1810764. PMID 32212520. S2CID 214644045.
- ^ a b Velarde MC, Bucu ME, Habana MA (November 2023). "Endometriosis as a highly relevant yet neglected gynecologic condition in Asian women". Endocrine Connections. 12 (11): e230169. doi:10.1530/EC-23-0169. PMC 10563646. PMID 37676242. "Compared with Caucasian women, Asian women are more likely to be diagnosed with endometriosis (odds ratio (OR) 1.63, 95% CI 1.03–2.58) (14). Filipinos, Indians, Japanese, and Koreans are among the top Asian ethnicities who are more likely to have endometriosis than Caucasian women (17)."
- ^ Chantalat E, Valera MC, Vaysse C, Noirrit E, Rusidze M, Weyl A, et al. (April 2020). "Estrogen Receptors and Endometriosis". International Journal of Molecular Sciences. 21 (8). MDPI AG: 2815. doi:10.3390/ijms21082815. PMC 7215544. PMID 32316608.
These mechanisms might act in unison to cause endometriosis, but the main trophic factor in endometriosis is estrogen and estrogen exposure plays a crucial role in the development of the disease via estrogen receptors (ERs) [1].
- ^ a b Gleicher N, el-Roeiy A, Confino E, Friberg J (July 1987). "Is endometriosis an autoimmune disease?". Obstetrics and Gynecology. 70 (1): 115–22. PMID 3110710.
- ^ a b c Giudice LC (June 2010). "Clinical practice. Endometriosis". The New England Journal of Medicine. 362 (25): 2389–98. doi:10.1056/NEJMcp1000274. PMC 3108065. PMID 20573927.
- ^ Koninckx PR, Ussia A, Mashiach R, Vilos G, Martin DC (September 2021). "Endometriosis Can Cause Pain at a Distance". Journal of Obstetrics and Gynaecology Canada. 43 (9). Elsevier BV: 1035–1036. doi:10.1016/j.jogc.2021.06.002. PMID 34481578. S2CID 237422801.
- ^ a b Stratton P, Berkley KJ (2011). "Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications". Human Reproduction Update. 17 (3): 327–46. doi:10.1093/humupd/dmq050. PMC 3072022. PMID 21106492.
- ^ Colette S, Donnez J (July 2011). "Are aromatase inhibitors effective in endometriosis treatment?". Expert Opinion on Investigational Drugs. 20 (7): 917–31. doi:10.1517/13543784.2011.581226. PMID 21529311. S2CID 19463907.
- ^ a b c Gałczyński K, Jóźwik M, Lewkowicz D, Semczuk-Sikora A, Semczuk A (November 2019). "Ovarian endometrioma - a possible finding in adolescent girls and young women: a mini-review". Journal of Ovarian Research. 12 (1): 104. doi:10.1186/s13048-019-0582-5. PMC 6839067. PMID 31699129.
 Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ a b c "What are the symptoms of endometriosis?". National Institutes of Health. Retrieved 4 October 2018.
- ^ Brown J, Farquhar C (March 2014). "Endometriosis: an overview of Cochrane Reviews". The Cochrane Database of Systematic Reviews. 2014 (3): CD009590. doi:10.1002/14651858.cd009590.pub2. PMC 6984415. PMID 24610050.
- ^ a b Ballard K, Lane H, Hudelist G, Banerjee S, Wright J (June 2010). "Can specific pain symptoms help in the diagnosis of endometriosis? A cohort study of women with chronic pelvic pain". Fertility and Sterility. 94 (1): 20–7. doi:10.1016/j.fertnstert.2009.01.164. PMID 19342028.
- ^ [page needed]Murray MT, Pizzorno J (2012). The Encyclopedia of Natural Medicine (3rd ed.). New York, NY: Simon and Schuster.
- ^ Asante A, Taylor RN (2011). "Endometriosis: the role of neuroangiogenesis". Annual Review of Physiology. 73: 163–82. doi:10.1146/annurev-physiol-012110-142158. PMID 21054165.
- ^ "Treatment of infertility in women with endometriosis". uptodate.com. Retrieved 18 December 2017.
- ^ Wolthuis AM, Meuleman C, Tomassetti C, D'Hooghe T, de Buck van Overstraeten A, D'Hoore A (November 2014). "Bowel endometriosis: colorectal surgeon's perspective in a multidisciplinary surgical team". World Journal of Gastroenterology. 20 (42): 15616–23. doi:10.3748/wjg.v20.i42.15616. PMC 4229526. PMID 25400445.
- ^ Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. (April 2012). "Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies". The Lancet. Oncology. 13 (4): 385–94. doi:10.1016/S1470-2045(11)70404-1. PMC 3664011. PMID 22361336.
- ^ Audebert A (April 2005). "[Women with endometriosis: are they different from others?]" [Women with endometriosis: are they different from others?]. Gynécologie, Obstétrique & Fertilité (in French). 33 (4): 239–46. doi:10.1016/j.gyobfe.2005.03.010. PMID 15894210.
- ^ Rowlands IJ, Nagle CM, Spurdle AB, Webb PM (December 2011). "Gynecological conditions and the risk of endometrial cancer". Gynecologic Oncology. 123 (3): 537–41. doi:10.1016/j.ygyno.2011.08.022. PMID 21925719.
- ^ Rousset P, Rousset-Jablonski C, Alifano M, Mansuet-Lupo A, Buy JN, Revel MP (March 2014). "Thoracic endometriosis syndrome: CT and MRI features". Clinical Radiology. 69 (3): 323–330. doi:10.1016/j.crad.2013.10.014. PMID 24331768.
- ^ Sarofim M, Attwell-Heap A, Trautman J, Kwok A, Still A (November 2019). "Unusual case of acute large bowel obstruction: endometriosis mimicking sigmoid malignancy". ANZ Journal of Surgery. 89 (11): E542–E543. doi:10.1111/ans.14869. PMID 30277298. S2CID 52902719.
- ^ Reis FM, Coutinho LM, Vannuccini S, Luisi S, Petraglia F (January 2020). "Is Stress a Cause or a Consequence of Endometriosis?". Reproductive Sciences. 27 (1): 39–45. doi:10.1007/s43032-019-00053-0. PMID 32046437. S2CID 209896867.
- ^ Acosta S, Leandersson U, Svensson SE, Johnsen J (May 2001). "Fallbeskrivning. Endometrios orsakade kolonileus, uretärobstruktion och hypertoni" [A case report. Endometriosis caused colonic ileus, ureteral obstruction and hypertension]. Läkartidningen (in Swedish). 98 (18): 2208–2212. PMID 11402601.
- ^ Ueda Y, Enomoto T, Miyatake T, Fujita M, Yamamoto R, Kanagawa T, et al. (June 2010). "A retrospective analysis of ovarian endometriosis during pregnancy". Fertility and Sterility. 94 (1): 78–84. doi:10.1016/j.fertnstert.2009.02.092. PMID 19356751.
- ^ Visouli AN, Zarogoulidis K, Kougioumtzi I, Huang H, Li Q, Dryllis G, et al. (October 2014). "Catamenial pneumothorax". Journal of Thoracic Disease. 6 (Suppl 4): S448-60. doi:10.3978/j.issn.2072-1439.2014.08.49. PMC 4203986. PMID 25337402.
- ^ a b c McCann MR, Schenk WB, Nassar A, Maimone S (September 2020). "Thoracic endometriosis presenting as a catamenial hemothorax with discordant video-assisted thoracoscopic surgery". Radiology Case Reports. 15 (9): 1419–1422. doi:10.1016/j.radcr.2020.05.064. PMC 7334551. PMID 32642009.
- ^ Wise J (April 2016). "Women with endometriosis show higher risk for heart disease". BMJ. 353: i1851. doi:10.1136/bmj.i1851. PMID 27036948. S2CID 28699291.
- ^ a b Saraswat L (2015). "ESHRE2015: Endometriosis associated with a greater risk of complications in pregnancy". endometriosis.org. European Society of Human Reproduction and Embryology. Retrieved 14 February 2024.
- ^ Schliep KC, Farland LV, Pollack AZ, Buck Louis G, Stanford JB, Allen-Brady K, et al. (November 2022). "Endometriosis diagnosis, staging and typology and adverse pregnancy outcome history". Paediatric and Perinatal Epidemiology. 36 (6): 771–781. doi:10.1111/ppe.12887. PMC 9588543. PMID 35570746.
- ^ a b Berlac JF, Hartwell D, Skovlund CW, Langhoff-Roos J, Lidegaard Ø (June 2017). "Endometriosis increases the risk of obstetrical and neonatal complications". Acta Obstetricia et Gynecologica Scandinavica. 96 (6): 751–760. doi:10.1111/aogs.13111. PMID 28181672.
- ^ Kvaskoff M, Mahamat-Saleh Y, Farland LV, Shigesi N, Terry KL, Harris HR, et al. (February 2021). "Endometriosis and cancer: a systematic review and meta-analysis". Human Reproduction Update. 27 (2). Oxford University Press (OUP): 393–420. doi:10.1093/humupd/dmaa045. hdl:20.500.11820/fa3c779d-3cc7-4d0d-b93a-d7176fd8244d. PMID 33202017.
- ^ Gandhi J, Wilson AL, Liang R, Weissbart SJ, Khan SA (11 November 2020). "Sciatic endometriosis: A narrative review of an unusual neurogynecologic condition". Journal of Endometriosis and Pelvic Pain Disorders. 13 (1). SAGE Publications: 3–9. doi:10.1177/2284026520970813. ISSN 2284-0265. S2CID 228834273.
- ^ Jia SZ, Leng JH, Shi JH, Sun PR, Lang JH (October 2012). "Health-related quality of life in women with endometriosis: a systematic review". Journal of Ovarian Research. 5 (1): 29. doi:10.1186/1757-2215-5-29. PMC 3507705. PMID 23078813.
- ^ Low WY, Edelmann RJ, Sutton C (February 1993). "A psychological profile of endometriosis patients in comparison to patients with pelvic pain of other origins". Journal of Psychosomatic Research. 37 (2): 111–116. doi:10.1016/0022-3999(93)90077-S. PMID 8463987.
- ^ a b c d e f g Fauser BC, Diedrich K, Bouchard P, Domínguez F, Matzuk M, Franks S, et al. (2011). "Contemporary genetic technologies and female reproduction". Human Reproduction Update. 17 (6): 829–47. doi:10.1093/humupd/dmr033. PMC 3191938. PMID 21896560.
- ^ Bischoff F, Simpson JL (April 2004). "Genetics of endometriosis: heritability and candidate genes". Best Practice & Research. Clinical Obstetrics & Gynaecology. 18 (2): 219–232. doi:10.1016/j.bpobgyn.2004.01.004. PMID 15157639.
- ^ Kapoor D, Davila W (2005). Endometriosis, Archived 11 November 2007 at the Wayback Machine eMedicine.
- ^ Giudice LC, Kao LC (2004). "Endometriosis". Lancet. 364 (9447): 1789–1799. doi:10.1016/S0140-6736(04)17403-5. PMID 15541453. S2CID 208788714.
- ^ Montgomery GW, Mortlock S, Giudice LC (February 2020). "Should Genetics Now Be Considered the Pre-eminent Etiologic Factor in Endometriosis?". Journal of Minimally Invasive Gynecology. 27 (2): 280–286. doi:10.1016/j.jmig.2019.10.020. PMC 7863762. PMID 31683028.
- ^ Vassilopoulou L, Matalliotakis M, Zervou MI, Matalliotaki C, Krithinakis K, Matalliotakis I, et al. (May 2019). "Defining the genetic profile of endometriosis". Experimental and Therapeutic Medicine. 17 (5): 3267–3281. doi:10.3892/etm.2019.7346. PMC 6447774. PMID 30988702.
- ^ Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT (September 2014). "Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets". Human Reproduction Update. 20 (5): 702–716. doi:10.1093/humupd/dmu015. PMC 4132588. PMID 24676469.
- ^ "MUC16 mucin 16, cell surface associated [Homo sapiens (human)] - Gene - NCBI". ncbi.nlm.nih.gov. Retrieved 13 November 2018.
- ^ "FN1 fibronectin 1 [Homo sapiens (human)] - Gene - NCBI". ncbi.nlm.nih.gov. Retrieved 13 November 2018.
- ^ Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De Vivo I, et al. (May 2017). "Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism". Nature Communications. 8 (1). Springer Science and Business Media LLC: 15539. Bibcode:2017NatCo...815539S. doi:10.1038/ncomms15539. PMC 5458088. PMID 28537267.
- ^ "GeneCards®: The Human Gene Database". www.genecards.org. Weizmann Institute of Science. Retrieved 7 February 2024.
- ^ Treloar SA, Bell TA, Nagle CM, Purdie DM, Green AC (June 2010). "Early menstrual characteristics associated with subsequent diagnosis of endometriosis". American Journal of Obstetrics and Gynecology. 202 (6): 534.e1–6. doi:10.1016/j.ajog.2009.10.857. PMID 20022587.[permanent dead link]
- ^ Nnoaham KE, Webster P, Kumbang J, Kennedy SH, Zondervan KT (September 2012). "Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies". Fertility and Sterility. 98 (3): 702–712.e6. doi:10.1016/j.fertnstert.2012.05.035. PMC 3502866. PMID 22728052.
- ^ Anger DL, Foster WG (January 2008). "The link between environmental toxicant exposure and endometriosis". Frontiers in Bioscience. 13 (13): 1578–93. doi:10.2741/2782. PMID 17981650. S2CID 12813384.
- ^ Guo SW (2004). "The link between exposure to dioxin and endometriosis: a critical reappraisal of primate data". Gynecologic and Obstetric Investigation. 57 (3): 157–73. doi:10.1159/000076374. PMID 14739528. S2CID 29701466.
- ^ Guo SW, Simsa P, Kyama CM, Mihályi A, Fülöp V, Othman EE, et al. (October 2009). "Reassessing the evidence for the link between dioxin and endometriosis: from molecular biology to clinical epidemiology". Molecular Human Reproduction. 15 (10): 609–24. doi:10.1093/molehr/gap075. PMID 19744969.
- ^ a b c Ahn C, Jeung EB (March 2023). "Endocrine-Disrupting Chemicals and Disease Endpoints". International Journal of Molecular Sciences. 24 (6): 5342. doi:10.3390/ijms24065342. PMC 10049097. PMID 36982431.
- ^ a b Rumph JT, Stephens VR, Archibong AE, Osteen KG, Bruner-Tran KL (2020). "Environmental Endocrine Disruptors and Endometriosis". Advances in Anatomy, Embryology, and Cell Biology. Advances in Anatomy, Embryology and Cell Biology. Vol. 232. pp. 57–78. doi:10.1007/978-3-030-51856-1_4. ISBN 978-3-030-51855-4. PMC 7978485. PMID 33278007.
- ^ a b Jiang I, Yong PJ, Allaire C, Bedaiwy MA (May 2021). "Intricate Connections between the Microbiota and Endometriosis". International Journal of Molecular Sciences. 22 (11): 5644. doi:10.3390/ijms22115644. PMC 8198999. PMID 34073257.
- ^ Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, et al. (April 2018). "Bacterial contamination hypothesis: a new concept in endometriosis". Reproductive Medicine and Biology. 17 (2): 125–133. doi:10.1002/rmb2.12083. PMC 5902457. PMID 29692669.
- ^ a b c d e f van der Linden PJ (November 1996). "Theories on the pathogenesis of endometriosis". Human Reproduction. 11 (Suppl 3): 53–65. doi:10.1093/humrep/11.suppl_3.53. PMID 9147102.
- ^ a b c d Hufnagel D, Li F, Cosar E, Krikun G, Taylor HS (September 2015). "The Role of Stem Cells in the Etiology and Pathophysiology of Endometriosis". Seminars in Reproductive Medicine. 33 (5): 333–40. doi:10.1055/s-0035-1564609. PMC 4986990. PMID 26375413.
- ^ Koninckx PR, Barlow D, Kennedy S (1999). "Implantation versus infiltration: the Sampson versus the endometriotic disease theory". Gynecologic and Obstetric Investigation. 47 (Supplement 1): 3–9, discussion 9–10. doi:10.1159/000052853. PMID 10087422. S2CID 29718095.
- ^ Malvezzi H, Marengo EB, Podgaec S, Piccinato CA (August 2020). "Endometriosis: current challenges in modeling a multifactorial disease of unknown etiology". Journal of Translational Medicine. 18 (1). Springer Science and Business Media LLC: 311. doi:10.1186/s12967-020-02471-0. PMC 7425005. PMID 32787880.
- ^ Pinkert TC, Catlow CE, Straus R (April 1979). "Endometriosis of the urinary bladder in a man with prostatic carcinoma". Cancer. 43 (4): 1562–7. doi:10.1002/1097-0142(197904)43:4<1562::aid-cncr2820430451>3.0.co;2-w. PMID 445352.
- ^ a b Signorile PG, Baldi F, Bussani R, D'Armiento M, De Falco M, Baldi A (April 2009). "Ectopic endometrium in human foetuses is a common event and sustains the theory of müllerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer". Journal of Experimental & Clinical Cancer Research. 28 (1): 49. doi:10.1186/1756-9966-28-49. PMC 2671494. PMID 19358700.
- ^ Mok-Lin EY, Wolfberg A, Hollinquist H, Laufer MR (February 2010). "Endometriosis in a patient with Mayer-Rokitansky-Küster-Hauser syndrome and complete uterine agenesis: evidence to support the theory of coelomic metaplasia". Journal of Pediatric and Adolescent Gynecology. 23 (1): e35-7. doi:10.1016/j.jpag.2009.02.010. PMID 19589710.
- ^ a b Marsh EE, Laufer MR (March 2005). "Endometriosis in premenarcheal girls who do not have an associated obstructive anomaly". Fertility and Sterility. 83 (3): 758–60. doi:10.1016/j.fertnstert.2004.08.025. PMID 15749511.
- ^ Thibodeau LL, Prioleau GR, Manuelidis EE, Merino MJ, Heafner MD (April 1987). "Cerebral endometriosis. Case report". Journal of Neurosurgery. 66 (4): 609–10. doi:10.3171/jns.1987.66.4.0609. PMID 3559727.
- ^ Rodman MH, Jones CW (April 1962). "Catamenial hemoptysis due to bronchial endometriosis". The New England Journal of Medicine. 266 (16): 805–8. doi:10.1056/nejm196204192661604. PMID 14493132.
- ^ Capellino S, Montagna P, Villaggio B, Sulli A, Soldano S, Ferrero S, et al. (June 2006). "Role of estrogens in inflammatory response: expression of estrogen receptors in peritoneal fluid macrophages from endometriosis". Annals of the New York Academy of Sciences. 1069 (1): 263–7. Bibcode:2006NYASA1069..263C. doi:10.1196/annals.1351.024. PMID 16855153. S2CID 35601442.
- ^ a b Young VJ, Brown JK, Saunders PT, Horne AW (2013). "The role of the peritoneum in the pathogenesis of endometriosis". Human Reproduction Update. 19 (5): 558–69. doi:10.1093/humupd/dmt024. PMID 23720497.
- ^ Redwine DB (October 2002). "Was Sampson wrong?". Fertility and Sterility. 78 (4): 686–93. doi:10.1016/S0015-0282(02)03329-0. PMID 12372441.
- ^ Sampson JA (March 1927). "Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation". Am. J. Pathol. 3 (2): 93–110.43. PMC 1931779. PMID 19969738.
- ^ a b Sampson JA (1927). "Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity". Am J Obstet Gynecol. 14 (4): 422–469. doi:10.1016/S0002-9378(15)30003-X.
- ^ Bruner-Tran KL, Yeaman GR, Crispens MA, Igarashi TM, Osteen KG (May 2008). "Dioxin may promote inflammation-related development of endometriosis". Fertility and Sterility. 89 (5 Suppl): 1287–98. doi:10.1016/j.fertnstert.2008.02.102. PMC 2430157. PMID 18394613.
- ^ Yuk JS, Shin JS, Shin JY, Oh E, Kim H, Park WI (2015). "Nickel Allergy Is a Risk Factor for Endometriosis: An 11-Year Population-Based Nested Case-Control Study". PLOS ONE. 10 (10): e0139388. Bibcode:2015PLoSO..1039388Y. doi:10.1371/journal.pone.0139388. PMC 4594920. PMID 26439741.
- ^ Soave I, Caserta D, Wenger JM, Dessole S, Perino A, Marci R (2015). "Environment and Endometriosis: a toxic relationship". European Review for Medical and Pharmacological Sciences. 19 (11): 1964–72. PMID 26125255.
- ^ Wellbery C (October 1999). "Diagnosis and treatment of endometriosis". American Family Physician. 60 (6). American Academy of Family Physicians: 1753–62, 1767–8. PMID 10537390. Archived from the original on 6 June 2011. Retrieved 26 July 2011.
- ^ Laschke MW, Giebels C, Menger MD (2011). "Vasculogenesis: a new piece of the endometriosis puzzle". Human Reproduction Update. 17 (5): 628–36. doi:10.1093/humupd/dmr023. PMID 21586449.
- ^ Morotti M, Vincent K, Brawn J, Zondervan KT, Becker CM (2014). "Peripheral changes in endometriosis-associated pain". Human Reproduction Update. 20 (5): 717–36. doi:10.1093/humupd/dmu021. PMC 4337970. PMID 24859987.
- ^ Yuk JS, Park EJ, Seo YS, Kim HJ, Kwon SY, Park WI (March 2016). "Graves Disease Is Associated With Endometriosis: A 3-Year Population-Based Cross-Sectional Study". Medicine. 95 (10): e2975. doi:10.1097/MD.0000000000002975. PMC 4998884. PMID 26962803.
- ^ Giudice LC, Kao LC (2004). "Endometriosis". Lancet. 364 (9447): 1789–99. doi:10.1016/S0140-6736(04)17403-5. PMID 15541453. S2CID 208788714.
- ^ a b c d e f Scutiero G, Iannone P, Bernardi G, Bonaccorsi G, Spadaro S, Volta CA, et al. (2017). "Oxidative Stress and Endometriosis: A Systematic Review of the Literature". Oxidative Medicine and Cellular Longevity. 2017: 7265238. doi:10.1155/2017/7265238. PMC 5625949. PMID 29057034.
- ^ a b c d Uzunçakmak C, Güldaş A, Ozçam H, Dinç K (2013). "Scar endometriosis: a case report of this uncommon entity and review of the literature". Case Reports in Obstetrics and Gynecology. 2013: 386783. doi:10.1155/2013/386783. PMC 3665185. PMID 23762683.
- ^ Weed JC, Ray JE (May 1987). "Endometriosis of the bowel". Obstetrics and Gynecology. 69 (5): 727–30. PMID 3574800.
- ^ a b Van den Bosch T, Van Schoubroeck D (August 2018). "Ultrasound diagnosis of endometriosis and adenomyosis: State of the art". Best Practice & Research. Clinical Obstetrics & Gynaecology. 51: 16–24. doi:10.1016/j.bpobgyn.2018.01.013. PMID 29506961. S2CID 3759091.
- ^ Beckman EN, Pintado SO, Leonard GL, Sternberg WH (May 1985). "Endometriosis of the prostate". The American Journal of Surgical Pathology. 9 (5): 374–379. doi:10.1097/00000478-198505000-00008. PMID 2418693.
- ^ Coleman-Belin J, Amakiri UO, Deng FM, Hoskoppal D, Safer JD, Reisman T (May 2024). "Hematospermia in a Transgender Woman with Evidence for Endometrial Tissue in the Prostate". AACE Clinical Case Reports. 10 (3): 80–83. doi:10.1016/j.aace.2024.01.006. PMC 11127599. PMID 38799045.
- ^ Jabr FI, Mani V (October 2014). "An unusual cause of abdominal pain in a male patient: Endometriosis". Avicenna Journal of Medicine. 4 (4): 99–101. doi:10.4103/2231-0770.140660. PMC 4183904. PMID 25298953.
- ^ Dwivedi AJ, Agrawal SN, Silva YJ (February 2002). "Abdominal wall endometriomas". Digestive Diseases and Sciences. 47 (2): 456–461. doi:10.1023/a:1013711314870. PMID 11855568. S2CID 7362461.
- ^ Kaunitz A, Di Sant'Agnese PA (December 1979). "Needle tract endometriosis: an unusual complication of amniocentesis". Obstetrics and Gynecology. 54 (6): 753–755. PMID 160025.
- ^ Koger KE, Shatney CH, Hodge K, McClenathan JH (September 1993). "Surgical scar endometrioma". Surgery, Gynecology & Obstetrics. 177 (3): 243–246. PMID 8356497.
- ^ Andres MP, Arcoverde FV, Souza CC, Fernandes LF, Abrão MS, Kho RM (February 2020). "Extrapelvic Endometriosis: A Systematic Review". Journal of Minimally Invasive Gynecology. 27 (2): 373–389. doi:10.1016/j.jmig.2019.10.004. PMID 31618674.
- ^ a b American Society For Reproductive (May 1997). "Revised American Society for Reproductive Medicine classification of endometriosis: 1996". Fertility and Sterility. 67 (5): 817–21. doi:10.1016/S0015-0282(97)81391-X. PMID 9130884.
- ^ a b Pugsley Z, Ballard K (June 2007). "Management of endometriosis in general practice: the pathway to diagnosis". The British Journal of General Practice. 57 (539): 470–6. PMC 2078174. PMID 17550672.
- ^ Zhang X, He T, Shen W (October 2020). "Comparison of physical examination, ultrasound techniques and magnetic resonance imaging for the diagnosis of deep infiltrating endometriosis: A systematic review and meta-analysis of diagnostic accuracy studies". Experimental and Therapeutic Medicine. 20 (4). Spandidos Publications: 3208–3220. doi:10.3892/etm.2020.9043. PMC 7444323. PMID 32855690.
- ^ a b c d e Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML (February 2016). "Imaging modalities for the non-invasive diagnosis of endometriosis". The Cochrane Database of Systematic Reviews. 2016 (2): CD009591. doi:10.1002/14651858.cd009591.pub2. PMC 7100540. PMID 26919512.
- ^ Chapron C, Marcellin L, Borghese B, Santulli P (November 2019). "Rethinking mechanisms, diagnosis and management of endometriosis". Nat Rev Endocrinol. 15 (11): 666–682. doi:10.1038/s41574-019-0245-z. PMID 31488888. S2CID 201838966.
- ^ "Reclassifying endometriosis as a syndrome would benefit patient care - The BMJ". 11 August 2020. Retrieved 17 August 2020.
- ^ a b c d e Johnson NP, Hummelshoj L (June 2013). "Consensus on current management of endometriosis". Human Reproduction. 28 (6): 1552–68. doi:10.1093/humrep/det050. PMID 23528916.
- ^ a b c Hsu AL, Khachikyan I, Stratton P (June 2010). "Invasive and noninvasive methods for the diagnosis of endometriosis". Clin Obstet Gynecol. 53 (2): 413–9. doi:10.1097/GRF.0b013e3181db7ce8. PMC 2880548. PMID 20436318.
- ^ Nisolle M, Paindaveine B, Bourdon A, Berlière M, Casanas-Roux F, Donnez J (June 1990). "Histologic study of peritoneal endometriosis in infertile women". Fertility and Sterility. 53 (6): 984–8. doi:10.1016/s0015-0282(16)53571-7. PMID 2351237.
- ^ Practice Committee of the American Society for Reproductive Medicine (April 2014). "Treatment of pelvic pain associated with endometriosis: a committee opinion". Fertility and Sterility. 101 (4): 927–35. doi:10.1016/j.fertnstert.2014.02.012. PMID 24630080.
- ^ a b c d e f "Endometriosis – Diagnosis, treatment and patient experiences". Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU). 4 May 2018. Retrieved 13 June 2018.
- ^ a b Fang J, Piessens S (June 2018). "A step-by-step guide to sonographic evaluation of deep infiltrating endometriosis". Sonography. 5 (2): 67–75. doi:10.1002/sono.12149.
- ^ a b Wild M, Pandhi S, Rendle J, Swift I, Ofuasia E (October 2020). "MRI for the diagnosis and staging of deeply infiltrating endometriosis: a national survey of BSGE accredited endometriosis centres and review of the literature". Br J Radiol. 93 (1114): 20200690. doi:10.1259/bjr.20200690. PMC 7548358. PMID 32706984.
- ^ Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG (January 2007). "Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients". Human Reproduction. 22 (1): 266–71. doi:10.1093/humrep/del339. PMID 16936305.
- ^ a b c d e May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM (2010). "Peripheral biomarkers of endometriosis: a systematic review". Human Reproduction Update. 16 (6): 651–74. doi:10.1093/humupd/dmq009. PMC 2953938. PMID 20462942.
- ^ a b Hirsch M, Duffy J, Davis CJ, Nieves Plana M, Khan KS (October 2016). "Diagnostic accuracy of cancer antigen 125 for endometriosis: a systematic review and meta-analysis". BJOG. 123 (11): 1761–8. doi:10.1111/1471-0528.14055. PMID 27173590. S2CID 22744182.
- ^ May KE, Villar J, Kirtley S, Kennedy SH, Becker CM (2011). "Endometrial alterations in endometriosis: a systematic review of putative biomarkers". Human Reproduction Update. 17 (5): 637–53. doi:10.1093/humupd/dmr013. PMID 21672902.
- ^ Gupta D, Hull ML, Fraser I, Miller L, Bossuyt PM, Johnson N, et al. (April 2016). "Endometrial biomarkers for the non-invasive diagnosis of endometriosis". The Cochrane Database of Systematic Reviews. 2016 (4): CD012165. doi:10.1002/14651858.CD012165. PMC 6953323. PMID 27094925.
- ^ Taghavipour M, Sadoughi F, Mirzaei H, Yousefi B, Moazzami B, Chaichian S, et al. (2020). "Apoptotic functions of microRNAs in pathogenesis, diagnosis, and treatment of endometriosis". Cell & Bioscience. 10: 12. doi:10.1186/s13578-020-0381-0. PMC 7014775. PMID 32082539.
- ^ Han L, Garcia R, Busca A, Parra-Herran C (November 2023). Turashvili G, Skala SL (eds.). "Ovary - nontumor - Nonneoplastic cysts / other - Endometriosis". Pathology Outlines.
- ^ McMaster-Fay R, Osborn R, Chandraratnam E. The Clinical Utility Of CD10 Immunohistochemical Staining In The Diagnosis Of Endometriosis (PDF). 10th World Congress of Endometriosis. Melbourne, Australia. Archived (PDF) from the original on 2 May 2013. Retrieved 18 July 2013.
- ^ Bourdel N, Alves J, Pickering G, Ramilo I, Roman H, Canis M (2014). "Systematic review of endometriosis pain assessment: how to choose a scale?". Human Reproduction Update. 21 (1): 136–52. doi:10.1093/humupd/dmu046. PMID 25180023.
- ^ "Endometriosis". www.who.int. Retrieved 24 November 2023.
- ^ "What are the treatments for endometriosis". Eunice Kennedy Shriver National Institute of Child Health and Human Development. Archived from the original on 3 August 2013. Retrieved 20 August 2013.
- ^ Moen MH, Rees M, Brincat M, Erel T, Gambacciani M, Lambrinoudaki I, et al. (September 2010). "EMAS position statement: Managing the menopause in women with a past history of endometriosis". Maturitas. 67 (1): 94–7. doi:10.1016/j.maturitas.2010.04.018. PMID 20627430.
- ^ a b c d Wellbery C (October 1999). "Diagnosis and treatment of endometriosis". American Family Physician. 60 (6): 1753–62, 1767–8. PMID 10537390. Archived from the original on 29 October 2013.
- ^ "Update on pharmacologic treatment for endometriosis- related pain". Women's Healthcare. 7 June 2020. Retrieved 3 October 2021.
- ^ Bafort C, Beebeejaun Y, Tomassetti C, Bosteels J, Duffy JM (October 2020). "Laparoscopic surgery for endometriosis". The Cochrane Database of Systematic Reviews. 2020 (10). Wiley: CD011031. doi:10.1002/14651858.cd011031.pub3. PMC 8428328. PMID 33095458.
- ^ a b c Vercellini P, Viganò P, Somigliana E, Fedele L (May 2014). "Endometriosis: pathogenesis and treatment". Nature Reviews. Endocrinology. 10 (5). Springer Science and Business Media LLC: 261–75. doi:10.1038/nrendo.2013.255. PMID 24366116. S2CID 13050344.
- ^ Speroff L, Glass RH, Kase NG (1999). Clinical Gynecologic Endocrinology and Infertility (6th ed.). Lippincott Willimas Wilkins. p. 1057. ISBN 0-683-30379-1.
- ^ "Endometriosis and Infertility: Can Surgery Help?" (PDF). American Society for Reproductive Medicine. 2008. Archived (PDF) from the original on 11 October 2010. Retrieved 31 October 2010.
- ^ "UNC Center for Endometriosis". UNC Department of Obstetrics & Gynecology. Archived from the original on 14 July 2021. Retrieved 14 July 2021.
- ^ Pundir J, Omanwa K, Kovoor E, Pundir V, Lancaster G, Barton-Smith P (2017). "Laparoscopic Excision Versus Ablation for Endometriosis-associated Pain: An Updated Systematic Review and Meta-analysis". Journal of Minimally Invasive Gynecology. 24 (5): 747–756. doi:10.1016/j.jmig.2017.04.008. PMID 28456617.
- ^ Muraskin A (16 July 2023). "Endometriosis, a painful and often overlooked disease, gets attention in a new film". NPR.
- ^ Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, De Bie B, et al. (March 2014). "ESHRE guideline: management of women with endometriosis". Human Reproduction. 29 (3). Oxford University Press (OUP): 400–12. doi:10.1093/humrep/det457. PMID 24435778.
- ^ Brunes, M, Altman, D, Pålsson, M, Söderberg, MW, Ek, M. Impact of hysterectomy on analgesic, psychoactive and neuroactive drug use in women with endometriosis: nationwide cohort study. BJOG 2021; 128: 846– 855. [1]
- ^ Selçuk İ, Bozdağ G (2013). "Recurrence of endometriosis; risk factors, mechanisms and biomarkers; review of the literature". J Turk Ger Gynecol Assoc. 14 (2): 98–103. doi:10.5152/jtgga.2013.52385. PMC 3881735. PMID 24592083.
- ^ Guo SW (2009). "Recurrence of endometriosis and its control". Human Reproduction Update. 15 (4): 441–61. doi:10.1093/humupd/dmp007. PMID 19279046.
- ^ Koninckx PR, Ussia A, Keckstein J, Adamyan LV, Zupi E, Wattiez A, et al. (2018). "Evidence-Based Medicine: Pandora's Box of Medical and Surgical Treatment of Endometriosis". Journal of Minimally Invasive Gynecology. 25 (3). Elsevier BV: 360–365. doi:10.1016/j.jmig.2017.11.012. PMID 29180308.
- ^ Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL (2001). "Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management". Digestive Surgery. 18 (4): 260–73. doi:10.1159/000050149. PMID 11528133. S2CID 30816909.
- ^ Trehan AK (2002). "Temporary ovarian suspension". Gynaecological Endoscopy. 11 (1): 309–314. doi:10.1046/j.1365-2508.2002.00520.x.
- ^ Abuzeid MI, Ashraf M, Shamma FN (February 2002). "Temporary ovarian suspension at laparoscopy for prevention of adhesions". The Journal of the American Association of Gynecologic Laparoscopists. 9 (1): 98–102. doi:10.1016/S1074-3804(05)60114-4. PMID 11821616.
- ^ Zorbas KA, Economopoulos KP, Vlahos NF (July 2015). "Continuous versus cyclic oral contraceptives for the treatment of endometriosis: a systematic review". Archives of Gynecology and Obstetrics. 292 (1): 37–43. doi:10.1007/s00404-015-3641-1. PMID 25644508. S2CID 23340983.
- ^ Brown J, Crawford TJ, Datta S, Prentice A (May 2018). "Oral contraceptives for pain associated with endometriosis". The Cochrane Database of Systematic Reviews. 2018 (5). Wiley: CD001019. doi:10.1002/14651858.cd001019.pub3. PMC 6494634. PMID 29786828.
- ^ Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S (2014). "Role of nuclear progesterone receptor isoforms in uterine pathophysiology". Human Reproduction Update. 21 (2): 155–73. doi:10.1093/humupd/dmu056. PMC 4366574. PMID 25406186.
- ^ "DANOCRINE : Brand of DANAZOL CAPSULES, USP" (PDF). Accessdata.fda.gov. Retrieved 3 March 2022.
- ^ a b D'Alterio MN, D'Ancona G, Raslan M, Tinelli R, Daniilidis A, Angioni S (April 2021). "Management Challenges of Deep Infiltrating Endometriosis". International Journal of Fertility & Sterility. 15 (2): 88–94. doi:10.22074/IJFS.2020.134689. PMC 8052801. PMID 33687160.
- ^ a b Brown J, Pan A, Hart RJ (December 2010). "Gonadotrophin-releasing hormone analogues for pain associated with endometriosis". The Cochrane Database of Systematic Reviews. 2010 (12): CD008475. doi:10.1002/14651858.CD008475.pub2. PMC 7388859. PMID 21154398.
- ^ Attar E, Bulun SE (May 2006). "Aromatase inhibitors: the next generation of therapeutics for endometriosis?". Fertility and Sterility. 85 (5): 1307–18. doi:10.1016/j.fertnstert.2005.09.064. PMID 16647373.
- ^ Słopień R, Męczekalski B (March 2016). "Aromatase inhibitors in the treatment of endometriosis". Przeglad Menopauzalny = Menopause Review. 15 (1). Termedia Sp. z.o.o.: 43–7. doi:10.5114/pm.2016.58773. PMC 4828508. PMID 27095958.
- ^ Garzon S, Laganà AS, Barra F, Casarin J, Cromi A, Raffaelli R, et al. (December 2020). "Aromatase inhibitors for the treatment of endometriosis: a systematic review about efficacy, safety and early clinical development". Expert Opinion on Investigational Drugs. 29 (12). Informa UK Limited: 1377–1388. doi:10.1080/13543784.2020.1842356. PMID 33096011. S2CID 225058751.
- ^ Fu J, Song H, Zhou M, Zhu H, Wang Y, Chen H, et al. (July 2017). "Progesterone receptor modulators for endometriosis". The Cochrane Database of Systematic Reviews. 2017 (7). Wiley: CD009881. doi:10.1002/14651858.cd009881.pub2. PMC 6483151. PMID 28742263.
- ^ Ness TJ (June 2013). "Not always lost in translation". Pain. 154 (6). Ovid Technologies (Wolters Kluwer Health): 775. doi:10.1016/j.pain.2013.03.022. PMID 23582150. S2CID 39113105.
- ^ Bayu P, Wibisono JJ (2024). "Vitamin C and E antioxidant supplementation may significantly reduce pain symptoms in endometriosis: A systematic review and meta-analysis of randomized controlled trials". PLOS ONE. 19 (5): e0301867. Bibcode:2024PLoSO..1901867B. doi:10.1371/journal.pone.0301867. PMC 11142610. PMID 38820340.
- ^ Flower A, Liu JP, Lewith G, Little P, Li Q (May 2012). "Chinese herbal medicine for endometriosis". The Cochrane Database of Systematic Reviews. 2012 (5): CD006568. doi:10.1002/14651858.CD006568.pub3. PMID 22592712.
- ^ Tiwari M (May 2017). "The role of serratiopeptidase in the resolution of inflammation". Asian Journal of Pharmaceutical Sciences. 12 (3): 209–215. doi:10.1016/j.ajps.2017.01.003. PMC 7032259. PMID 32104332.
- ^ Ethiraj S, Gopinath S (2017). "Production, purification, characterization, immobilization, and application of Serrapeptase: a review". Frontiers in Biology. 12 (5): 333–348. doi:10.1007/s11515-017-1461-3. S2CID 89694879.
- ^ a b Laschke MW, Menger MD (2012). "Anti-angiogenic treatment strategies for the therapy of endometriosis". Human Reproduction Update. 18 (6): 682–702. doi:10.1093/humupd/dms026. PMID 22718320.
- ^ Canny GO, Lessey BA (May 2013). "The role of lipoxin A4 in endometrial biology and endometriosis". Mucosal Immunology. 6 (3): 439–50. doi:10.1038/mi.2013.9. PMC 4062302. PMID 23485944.
- ^ Streuli I, de Ziegler D, Santulli P, Marcellin L, Borghese B, Batteux F, et al. (February 2013). "An update on the pharmacological management of endometriosis". Expert Opinion on Pharmacotherapy. 14 (3): 291–305. doi:10.1517/14656566.2013.767334. PMID 23356536. S2CID 10052884.
- ^ Grammatis AL, Georgiou EX, Becker CM (August 2021). "Pentoxifylline for the treatment of endometriosis-associated pain and infertility". The Cochrane Database of Systematic Reviews. 2021 (8): CD007677. doi:10.1002/14651858.CD007677.pub4. PMC 8407096. PMID 34431079. S2CID 237294362.
- ^ "Practice bulletin no. 114: management of endometriosis". Obstetrics and Gynecology. 116 (1): 223–36. July 2010. doi:10.1097/AOG.0b013e3181e8b073. PMID 20567196.
- ^ Brown J, Crawford TJ, Allen C, Hopewell S, Prentice A (January 2017). "Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis". The Cochrane Database of Systematic Reviews. 1 (1): CD004753. doi:10.1002/14651858.CD004753.pub4. PMC 6464974. PMID 28114727.
- ^ a b c Saunders PT, Horne AW (May 2021). "Endometriosis: Etiology, pathobiology, and therapeutic prospects". Cell. 184 (11). Elsevier BV: 2807–2824. doi:10.1016/j.cell.2021.04.041. hdl:20.500.11820/bb7ded31-cc3d-449e-a0dc-ce4b1a0531d2. PMID 34048704. S2CID 235226513.
- ^ Valiani M, Ghasemi N, Bahadoran P, Heshmat R (2010). "The effects of massage therapy on dysmenorrhea caused by endometriosis". Iranian Journal of Nursing and Midwifery Research. 15 (4): 167–71. PMC 3093183. PMID 21589790.
- ^ Samy A, Taher A, Sileem SA, Abdelhakim AM, Fathi M, Haggag H, et al. (January 2021). "Medical therapy options for endometriosis related pain, which is better? A systematic review and network meta-analysis of randomized controlled trials". Journal of Gynecology Obstetrics and Human Reproduction. 50 (1). Elsevier BV: 101798. doi:10.1016/j.jogoh.2020.101798. PMID 32479894. S2CID 219173190.
- ^ a b c d "Endometrios – diagnostik, behandling och bemötande". sbu.se (in Swedish). Statens beredning för medicinsk och social utvärdering (SBU); Swedish Agency for Health Technology Assessment and Assessment of Social Services. 4 May 2018. p. 121. Retrieved 13 June 2018.
- ^ a b [non-primary source needed] Wurn BF, Wurn LJ, Patterson K, King CR, Scharf ES (2011). "Decreasing dyspareunia and dysmenorrhea in women with endometriosis via a manual physical therapy: Results from two independent studies". Journal of Endometriosis and Pelvic Pain Disorders. 3 (4): 188–196. doi:10.5301/JE.2012.9088. PMC 6154826. Archived from the original on 29 October 2013.
- ^ Chen I, Veth VB, Choudhry AJ, Murji A, Zakhari A, Black AY, et al. (November 2020). "Pre- and postsurgical medical therapy for endometriosis surgery". The Cochrane Database of Systematic Reviews. 11 (12): CD003678. doi:10.1002/14651858.CD003678.pub3. PMC 8127059. PMID 33206374.
- ^ a b Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, et al. (August 2018). "Risk for and consequences of endometriosis: A critical epidemiologic review". Best Practice & Research. Clinical Obstetrics & Gynaecology. 51: 1–15. doi:10.1016/j.bpobgyn.2018.06.001. PMID 30017581. S2CID 51679656.
- ^ "Endometriosis Ultrasound: Procedure, Diagnosis, & Follow Up". Cleveland Clinic. Retrieved 7 March 2022.
- ^ Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, et al. (August 2011). "Incidence of endometriosis by study population and diagnostic method: the ENDO study". Fertil. Steril. 96 (2): 360–5. doi:10.1016/j.fertnstert.2011.05.087. PMC 3143230. PMID 21719000.
- ^ a b Brosens I (2012). Endometriosis: Science and Practice. John Wiley & Sons. p. 3. ISBN 978-1-4443-9849-6.
- ^ Nothnick WB (June 2011). "The emerging use of aromatase inhibitors for endometriosis treatment". Reproductive Biology and Endocrinology. 9: 87. doi:10.1186/1477-7827-9-87. PMC 3135533. PMID 21693036.
- ^ Bulun SE, Zeitoun K, Sasano H, Simpson ER (1999). "Aromatase in aging women". Seminars in Reproductive Endocrinology. 17 (4): 349–58. doi:10.1055/s-2007-1016244. PMID 10851574. S2CID 25628258.
- ^ Batt RE, Mitwally MF (December 2003). "Endometriosis from thelarche to midteens: pathogenesis and prognosis, prevention and pedagogy". Journal of Pediatric and Adolescent Gynecology. 16 (6): 337–47. doi:10.1016/j.jpag.2003.09.008. PMID 14642954.
- ^ a b Guo SW (11 March 2009). "Recurrence of endometriosis and its control". Human Reproduction Update. 15 (4): 441–61. doi:10.1093/humupd/dmp007. PMID 19279046.
- ^ Batt RE (2011). A history of endometriosis. London: Springer. pp. 13–38. doi:10.1007/978-0-85729-585-9. ISBN 978-0-85729-585-9.
- ^ a b c d e f g h i j Nezhat C, Nezhat F, Nezhat C (December 2012). "Endometriosis: ancient disease, ancient treatments". Fertility and Sterility. 98 (6 Suppl): S1-62. doi:10.1016/j.fertnstert.2012.08.001. PMID 23084567.
- ^ Meigs JV (November 1941). "Endometriosis—Its Significance". Ann. Surg. 114 (5): 866–74. doi:10.1097/00000658-194111000-00007. PMC 1385984. PMID 17857917.
- ^ Barbieri RL (January 1992). "Hormonal therapy of endometriosis". Infertility and Reproductive Medicine Clinics of North America. 3 (1): 187–200.
The hormonal therapy of endometriosis continues to evolve. In the 1940s and 1950s, high-dose testosterone and diethylstilbestrol regimens were the only hormonal agents available in the treatment of endometriosis. These agents, although efficacious, were associated with intolerable side effects. The current armamentarium of hormonal agents-the GnRH analogues, danazol, and the synthetic progestins-is efficacious and has fewer side effects.
- ^ a b c Aiman J (6 December 2012). Infertility: Diagnosis and Management. Springer Science & Business Media. pp. 261–. ISBN 978-1-4613-8265-2.
- ^ a b c d Josimovich JB (11 November 2013). Gynecologic Endocrinology. Springer Science & Business Media. pp. 387–. ISBN 978-1-4613-2157-6.
- ^ a b c d Kistner RW (1995). Kistner's Gynecology: Principles and Practice. Mosby. p. 263. ISBN 978-0-8151-7479-0.
- ^ Barra F, Grandi G, Tantari M, Scala C, Facchinetti F, Ferrero S (April 2019). "A comprehensive review of hormonal and biological therapies for endometriosis: latest developments". Expert Opin Biol Ther. 19 (4): 343–360. doi:10.1080/14712598.2019.1581761. PMID 30763525. S2CID 73455399.
- ^ Gallagher K (31 March 2021). "Irish songwriter Ruth Anne opens up on her debilitating fight with endometriosis". Irish Mirror. Retrieved 11 April 2023.
- ^ "Endometriosis showed me we need better ways to talk about women's pain | Emma Barnett". TheGuardian.com. 22 October 2020.
- ^ Hayden J (11 May 2020). "'Nearly broke me' Spice Girls' Emma Bunton describes struggling to conceive with endometriosis". Her.ie. Retrieved 28 May 2021.
- ^ "Alexa Chung Reveals Her Battle With Endometriosis—And Taps Into an Empowering Online Community". Vogue. 18 July 2019. Retrieved 11 April 2023.
- ^ "Champions Corner: Collins unleashes the best tennis of her career after life-changing surgery". Women's Tennis Association. Retrieved 3 March 2022.
- ^ "Watch: Olivia Culpo Shares Emotional Details of Her Endometriosis Journey". Sports Illustrated.
- ^ "Lena Dunham Gives Health Update Following Battle with Endometriosis". People. Retrieved 11 April 2023.
- ^ "Congresswoman Abby Finkenauer Opens Up About Her Struggle With Endometriosis". Glamour. 5 March 2020. Retrieved 17 December 2021.
- ^ "EXCLUSIVE: Bethenny Frankel in Tears Over Recent Health Scare: 'I Really Tried to Hold It All Together'". Entertainment Tonight. 22 June 2016. Retrieved 11 April 2023.
- ^ "Blossom Ball 2009 – Whoopi Goldberg". Endometriosis Foundation of America. 27 November 2007. Retrieved 21 January 2021.
- ^ "Radio presenter Mel Greig's shocking photo shows reality of living with endometriosis". News.com.au. 28 March 2018. Retrieved 21 January 2021.
- ^ Iasimone A (7 January 2017). "Halsey undergoes surgery to treat endometriosis". Billboard. Retrieved 27 November 2018.
- ^ "Emma Hayes: Chelsea manager has emergency hysterectomy because of endometriosis". BBC Sport. BBC. 13 October 2022. Retrieved 28 December 2022.
- ^ Murray R (9 September 2017). "Julianne Hough opens up about endometriosis: 'I just thought it was normal'". Today. Retrieved 21 January 2021.
- ^ "Julianne Hough Won't Let Endometriosis Stop Her From Having a Family With Brooks Laich: "We've Discussed Options"". Endometriosis : Causes - Symptoms - Diagnosis - and Treatment. 23 April 2018. Retrieved 31 December 2023.
- ^ Olya G (24 March 2017). "Julianne Hough Opens Up About Her Struggle with Endometriosis". Peoplemag. Retrieved 31 December 2023.
- ^ Hustwaite B (14 August 2018). "Endometriosis: The pain sucks, but so does just getting a diagnosis". Hack on Triple J. Retrieved 21 January 2021.
- ^ Dye J (8 March 2023). "On International Women's Day, Bindi Irwin reveals 10-year struggle with endometriosis". Australian Broadcasting Corporation News. Retrieved 10 March 2023.
- ^ "Why Jaime King Decided It Was Time to Talk About Her Years-Long Fertility Struggle". E! Online. 24 April 2022. Retrieved 11 April 2023.
- ^ "Padma Lakshmi shares her struggle with endometriosis". Redbook. 17 October 2011. Archived from the original on 2 November 2020. Retrieved 9 March 2021 – via YouTube.
- ^ Hajdu D (30 November 2012). "'80s Pop". The New York Times. ISSN 0362-4331. Retrieved 11 April 2023.
- ^ "No Kidding: Jillian Michaels is Not "Doing That" to Her Body". Bitch Media. 7 February 2011. Archived from the original on 11 April 2023. Retrieved 11 April 2023.
- ^ "Monica's Second Surgery For Her Endometriosis Was 'Very Hard' And She 'Ended Up Having Multiple Blood Transfusions'". MadameNoire. 9 June 2021. Retrieved 11 April 2023.
- ^ Wilson-Beevers H (28 September 2022). "Marilyn Monroe's battle with endometriosis is often ignored – but it's a vital part of her story". Cosmopolitan. Retrieved 11 April 2023.
- ^ Henderson W (14 June 2017). "Tia Mowry Releases a Cookbook for Women With Endometrosis". Endometriosis News. Retrieved 11 April 2023.
- ^ Devereux N (19 August 2015). "Sinead O'Connor tells of illness hell: "I'm in a bad way"". Retrieved 5 August 2023.
- ^ Vargas A. "12 celebrities who have opened up about having endometriosis". Insider. Retrieved 6 August 2021.
- ^ Scott E (12 June 2016). "Daisy Ridley opened up about her struggle with endometriosis". Cosmopolitan. Retrieved 29 June 2021.
- ^ "Emma Roberts shares how her undiagnosed endometriosis affected her pregnancy journey". Self. Condé Nast. 11 November 2020. Retrieved 15 July 2021.
- ^ "EFA2011: Susan Sarandon speaks up about endometriosis". Endometriosis.org. Retrieved 11 April 2023.
- ^ "Amy Schumer 'feeling good' after endometriosis surgery and liposuction". The Independent Tribune. 20 January 2022. Retrieved 11 April 2023.
- ^ "'General Hospital': Kirsten Storms opens up about return – The TV Guy – Orlando Sentinel". 25 December 2012. Archived from the original on 25 December 2012. Retrieved 18 October 2021.
- ^ Haller S. "Gabrielle Union says she probably can't get pregnant because of adenomyosis. What exactly is that?". USA Today. Retrieved 11 April 2023.
- ^ Staff Writer. "Another victim of the 'Dancing With The Stars' curse". The Columbus Dispatch. Retrieved 31 December 2023.
- ^ Richenthal M (30 October 2008). "Lacey Schwimmer is Also Sick". TV Fanatic. Retrieved 31 December 2023.
- ^ "Lena Dunham, Julianne Hough and More Who've Opened Up About Endometriosis Battles: "You Don't Have to Ignore Pain"". E! Online. 23 March 2017. Retrieved 31 December 2023.
- ^ Mazziotta J (5 February 2021). "Chrissy Teigen says endometriosis surgery was 'a toughie' but better than 'the pain of endo'". People. Retrieved 15 July 2021.
- ^ "Yellow Wiggle Emma Watkins opens up about the agony of endometriosis". PerthNow. 16 March 2019. Retrieved 21 January 2021.
- ^ "Mae Whitman: 'Endometriosis Is Like Being Shot With a Cannonball in the Stomach'". Glamour. 21 May 2020. Retrieved 11 April 2023.
- ^ "Jessica Williams on the 'Debilitating' Symptom That Led to Her Endometriosis Diagnosis". Self. 12 April 2022. Retrieved 11 April 2023.
- ^ Dervish-O'Kane R (27 December 2022). "Leah Williamson, captain of the Lionesses, is our January cover star". Women's Health. Hearst UK. Retrieved 28 December 2022.
- ^ a b Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL (December 2006). "Economic burden of endometriosis". Fertility and Sterility. 86 (6): 1561–72. doi:10.1016/j.fertnstert.2006.06.015. PMID 17056043. S2CID 20623034.
- ^ Koltermann KC, Dornquast C, Ebert AD, Reinhold T (2017). "Economic Burden of Endometriosis: A Systematic Review". Ann Reprod Med Treat. 2 (2m): 1015. S2CID 32839234.
- ^ Grundström H, Hammar Spagnoli G, Lövqvist L, Olovsson M (2020). "Healthcare Consumption and Cost Estimates Concerning Swedish Women with Endometriosis". Gynecologic and Obstetric Investigation. 85 (3): 237–244. doi:10.1159/000507326. PMID 32248191. S2CID 214811610.
- ^ Soliman AM, Coyne KS, Gries KS, Castelli-Haley J, Snabes MC, Surrey ES (July 2017). "The Effect of Endometriosis Symptoms on Absenteeism and Presenteeism in the Workplace and at Home". Journal of Managed Care & Specialty Pharmacy. 23 (7): 745–754. doi:10.18553/jmcp.2017.23.7.745. PMC 10398072. PMID 28650252.
- ^ As-Sanie S, Black R, Giudice LC, Gray Valbrun T, Gupta J, Jones B, et al. (August 2019). "Assessing research gaps and unmet needs in endometriosis". American Journal of Obstetrics and Gynecology. 221 (2): 86–94. doi:10.1016/j.ajog.2019.02.033. PMID 30790565. S2CID 73480251.
- ^ Hudelist G, Fritzer N, Thomas A, Niehues C, Oppelt P, Haas D, et al. (December 2012). "Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences". Human Reproduction. 27 (12): 3412–6. doi:10.1093/humrep/des316. PMID 22990516.
- ^ "Test d'auto-évaluation du JOGC". Journal of Obstetrics and Gynaecology Canada. 25 (12): 1046–1051. December 2003. doi:10.1016/s1701-2163(16)30350-4. ISSN 1701-2163.
- ^ Quibel A, Puscasiu L, Marpeau L, Roman H (June 2013). "[General practitioners and the challenge of endometriosis screening and care: results of a survey]". Gynécologie, Obstétrique & Fertilité. 41 (6): 372–80. doi:10.1016/j.gyobfe.2012.02.024. PMID 22521982.
- ^ a b Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL (April 2003). "Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women". Human Reproduction. 18 (4): 756–9. doi:10.1093/humrep/deg136. PMID 12660267.
- ^ Shade GH, Lane M, Diamond MP (24 June 2011). "Endometriosis in the African American woman—racially, a different entity?". Gynecological Surgery. 9: 59–62. doi:10.1007/s10397-011-0685-5. S2CID 6288739.
- ^ Hoffman KM, Trawalter S, Axt JR, Oliver MN (April 2016). "Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites". Proceedings of the National Academy of Sciences of the United States of America. 113 (16): 4296–301. Bibcode:2016PNAS..113.4296H. doi:10.1073/pnas.1516047113. PMC 4843483. PMID 27044069.
- ^ a b Matías-González Y, Sánchez-Galarza AN, Flores-Caldera I, Rivera-Segarra E (March 2021). ""Es que tú eres una changa": stigma experiences among Latina women living with endometriosis". Journal of Psychosomatic Obstetrics and Gynaecology. 42 (1): 67–74. doi:10.1080/0167482X.2020.1822807. PMC 8893272. PMID 32964770. S2CID 221862356.
- ^ Sims OT, Gupta J, Missmer SA, Aninye IO (August 2021). "Stigma and Endometriosis: A Brief Overview and Recommendations to Improve Psychosocial Well-Being and Diagnostic Delay". International Journal of Environmental Research and Public Health. 18 (15): 8210. doi:10.3390/ijerph18158210. PMC 8346066. PMID 34360501.
- ^ Kocas HD, Rubin LR, Lobel M (September 2023). "Stigma and mental health in endometriosis". European Journal of Obstetrics & Gynecology and Reproductive Biology. 19. Elsevier BV: 100228. doi:10.1016/j.eurox.2023.100228. PMC 10465859. PMID 37654520.
- ^ Henry JE (27 May 2022). "Period Stigma and the Unacknowledged System of Oppression". Stanford | Digital Education. Retrieved 24 November 2023.
- ^ Sims OT, Gupta J, Missmer SA, Aninye IO (August 2021). "Stigma and Endometriosis: A Brief Overview and Recommendations to Improve Psychosocial Well-Being and Diagnostic Delay". International Journal of Environmental Research and Public Health. 18 (15): 8210. doi:10.3390/ijerph18158210. PMC 8346066. PMID 34360501.
This article incorporates text in the public domain as a Swedish government "utterance" by URL§9