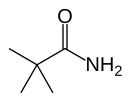
Pivalamide
Appearance
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,2-Dimethylpropanamide | |||
| Other names
Trimethylacetamide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.010.949 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H11NO | |||
| Molar mass | 101.149 g·mol−1 | ||
| Melting point | 154 to 157 °C (309 to 315 °F; 427 to 430 K) | ||
| Boiling point | 212 °C (414 °F; 485 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Pivalamide (2,2-dimethylpropanamide, or NDEPA), a simple amide substituted with a tert-butyl group having the chemical formula: tBu-CO-NH2. It is the amide of pivalic acid.
N-Pivalamide, is a functional group having the following chemical formula: tBu-CO-NH-R
References
[edit]- ^ Pivalamide, chemicalbook.com