Peatland



A peatland is a type of wetland whose soils consist of organic matter from decaying plants, forming layers of peat. Peatlands arise because of incomplete decomposition of organic matter, usually litter from vegetation, due to water-logging and subsequent anoxia.[1] Peatlands are unusual landforms that derive mostly from biological rather than physical processes, and can take on characteristic shapes and surface patterning.
The formation of peatlands is primarily controlled by climatic conditions such as precipitation and temperature, although terrain relief is a major factor as waterlogging occurs more easily on flatter ground and in basins.[2] Peat formation typically initiates as a paludification of a mineral soil forests, terrestrialisation of lakes, or primary peat formation on bare soils on previously glaciated areas.[3] A peatland that is actively forming peat is called a mire. All types of mires share the common characteristic of being saturated with water, at least seasonally with actively forming peat, while having their own ecosystem.[4]
Peatlands are the largest natural carbon store on land. Covering around 3 million km2 globally, they sequester 0.37 gigatons (Gt) of carbon dioxide (CO2) a year. Peat soils store over 600 Gt of carbon, more than the carbon stored in all other vegetation types, including forests. This substantial carbon storage represents about 30% of the world's soil carbon, underscoring their critical importance in the global carbon cycle.[5] In their natural state, peatlands provide a range of ecosystem services, including minimising flood risk and erosion, purifying water and regulating climate.[3][6]
Peatlands are under threat by commercial peat harvesting, drainage and conversion for agriculture (notably palm oil in the tropics) and fires, which are predicted to become more frequent with climate change. The destruction of peatlands results in release of stored greenhouse gases into the atmosphere, further exacerbating climate change.
Types
[edit]For botanists and ecologists, the term peatland is a general term for any terrain dominated by peat to a depth of at least 30 cm (12 in), even if it has been completely drained (i.e., a peatland can be dry). A peatland that is still capable of forming new peat is called a mire, while drained and converted peatlands might still have a peat layer but are not considered mires as the formation of new peat has ceased.[1]
There are two types of mire: bog and fen.[2] A bog is a mire that, due to its raised location relative to the surrounding landscape, obtains all its water solely from precipitation (ombrotrophic).[7] A fen is located on a slope, flat, or in a depression and gets most of its water from the surrounding mineral soil or from groundwater (minerotrophic). Thus, while a bog is always acidic and nutrient-poor, a fen may be slightly acidic, neutral, or alkaline, and either nutrient-poor or nutrient-rich.[8] All mires are initially fens when the peat starts to form, and may turn into bogs once the height of the peat layer reaches above the surrounding land. A quagmire or is a floating (quaking) mire, bog, or any peatland being in a stage of hydrosere or hydrarch (hydroseral) succession, resulting in pond-filling yields underfoot (floating mats). Ombrotrophic types of quagmire may be called quaking bog (quivering bog). Minerotrophic types can be named with the term quagfen.[9]
Some swamps can also be peatlands (e.g.: peat swamp forest), while marshes are generally not considered to be peatlands.[2] Swamps are characterized by their forest canopy or the presence of other tall and dense vegetation like papyrus. Like fens, swamps are typically of higher pH level and nutrient availability than bogs. Some bogs and fens can support limited shrub or tree growth on hummocks. A marsh is a type of wetland within which vegetation is rooted in mineral soil.
Global distribution
[edit]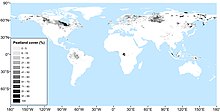
Peatlands are found around the globe, although are at their greatest extent at high latitudes in the Northern Hemisphere. Peatlands are estimated to cover around 3% of the globe's surface,[6] although estimating the extent of their cover worldwide is difficult due to the varying accuracy and methodologies of land surveys from many countries.[2] Mires occur wherever conditions are right for peat accumulation: largely where organic matter is constantly waterlogged. Hence the distribution of mires is dependent on topography, climate, parent material, biota and time.[10] The type of mire—bog, fen, marsh or swamp—depends also on each of these factors.
The largest accumulation of mires constitutes around 64% of global peatlands and is found in the temperate, boreal and subarctic zones of the Northern Hemisphere.[11] Mires are usually shallow in polar regions because of the slow rate of accumulation of dead organic matter, and often contain permafrost and palsas. Very large swathes of Canada, northern Europe and northern Russia are covered by boreal mires. In temperate zones mires are typically more scattered due to historical drainage and peat extraction, but can cover large areas. One example is blanket bog where precipitation is very high i.e., in maritime climates inland near the coasts of the north-east and south Pacific, and the north-west and north-east Atlantic. In the sub-tropics, mires are rare and restricted to the wettest areas.
Mires can be extensive in the tropics, typically underlying tropical rainforest (for example, in Kalimantan, the Congo Basin and Amazon basin). Tropical peat formation is known to occur in coastal mangroves as well as in areas of high altitude.[3] Tropical mires largely form where high precipitation is combined with poor conditions for drainage.[2] Tropical mires account for around 11% of peatlands globally (more than half of which can be found in Southeast Asia), and are most commonly found at low altitudes, although they can also be found in mountainous regions, for example in South America, Africa and Papua New Guinea.[11] Indonesia, particularly on the islands of Sumatra, Kalimantan and Papua, has one of the largest peatlands in the world, with an area of about 24 million hectares. These peatlands play an important role in global carbon storage and have very high biodiversity. However, peatlands in Indonesia also face major threats from deforestation and forest fires.[12] In the early 21st century, the world's largest tropical mire was found in the Central Congo Basin, covering 145,500 km2 and storing up to 1013 kg of carbon.[13]
The total area of mires has declined globally due to drainage for agriculture, forestry and peat harvesting. For example, more than 50% of the original European mire area which is more than 300,000 km2 has been lost.[14][clarification needed] Some of the largest losses have been in Russia, Finland, the Netherlands, the United Kingdom, Poland and Belarus. A catalog of the peat research collection at the University of Minnesota Duluth provides references to research on worldwide peat and peatlands.[15]
Biochemical processes
[edit]
Peatlands have unusual chemistry that influences, among other things, their biota and water outflow. Peat has very high cation-exchange capacity due to its high organic matter content: cations such as Ca2+ are preferentially adsorbed onto the peat in exchange for H+ ions. Water passing through peat declines in nutrients and pH. Therefore, mires are typically nutrient-poor and acidic unless the inflow of groundwater (bringing in supplementary cations) is high.[16]
Generally, whenever the inputs of carbon into the soil from dead organic matter exceed the carbon outputs via organic matter decomposition, peat is formed. This occurs due to the anoxic state of water-logged peat, which slows down decomposition.[17] Peat-forming vegetation is typically also recalcitrant (poorly decomposing) due to high lignin and low nutrient content.[18] Topographically, accumulating peat elevates the ground surface above the original topography. Mires can reach considerable heights above the underlying mineral soil or bedrock: peat depths of above 10 m have been commonly recorded in temperate regions (many temperate and most boreal mires were removed by ice sheets in the last Ice Age), and above 25 m in tropical regions.[7] When the absolute decay rate of peat in the catotelm (the lower, water-saturated zone of the peat layer) matches the rate of input of new peat into the catotelm, the mire will stop growing in height.[8]
Carbon storage and methanogenesis
[edit]Despite accounting for just 3% of Earth's land surfaces, peatlands are collectively a major carbon store containing between 500 and 700 billion tonnes of carbon. Carbon stored within peatlands equates to over half the amount of carbon found in the atmosphere.[3] Peatlands interact with the atmosphere primarily through the exchange of carbon dioxide, methane and nitrous oxide,[1] and can be damaged by excess nitrogen from agriculture or rainwater.[19] The sequestration of carbon dioxide takes place at the surface via the process of photosynthesis, while losses of carbon dioxide occur through living plants via autotrophic respiration and from the litter and peat via heterotrophic respiration.[2] In their natural state, mires are a small atmospheric carbon dioxide sink through the photosynthesis of peat vegetation, which outweighs their release of greenhouse gases. On the other hand, most mires are generally net emitters of methane and nitrous oxide.[20] Due to the continued CO2 sequestration over millennia, and because of the longer atmospheric lifespan of the CO2 molecules compared with methane and nitrous oxide, peatlands have had a net cooling effect on the atmosphere.[21]
The water table position of a peatland is the main control of its carbon release to the atmosphere. When the water table rises after a rainstorm, the peat and its microbes are submerged under water inhibiting access to oxygen, reducing CO2 release via respiration. Carbon dioxide release increases when the water table falls lower, such as during a drought, as this increases the availability of oxygen to the aerobic microbes thus accelerating peat decomposition.[22] Levels of methane emissions also vary with the water table position and temperature. A water table near the peat surface gives the opportunity for anaerobic microorganisms to flourish.
Methanogens are strictly anaerobic organisms and produce methane from organic matter in anoxic conditions below the water table level, while some of that methane is oxidised by methanotrophs above the water table level. Therefore, changes in water table level influence the size of these methane production and consumption zones. Increased soil temperatures also contribute to increased seasonal methane flux. A study in Alaska found that methane may vary by as much as 300% seasonally with wetter and warmer soil conditions due to climate change.[23]
Peatlands are important for studying past climate because they are sensitive to changes in the environment and can reveal levels of isotopes, pollutants, macrofossils, metals from the atmosphere and pollen.[24] For example, carbon-14 dating can reveal the age of the peat. The dredging and destruction of a peatland will release the carbon dioxide that could reveal irreplaceable information about the past climatic conditions. Many kinds of microorganisms inhabit peatlands, due to the regular supply of water and abundance of peat forming vegetation. These microorganisms include but are not limited to methanogens, algae, bacteria, zoobenthos, of which Sphagnum species are most abundant.[25]
Humic substances
[edit]Peat contains substantial organic matter, where humic acid dominates. Humic materials can store substantial amounts of water, making them an essential component in the peat environment, contributing to increased carbon storage due to the resulting anaerobic condition. If the peatland is dried from long-term cultivation and agricultural use, it will lower the water table, and the increased aeration will release carbon.[26] Upon extreme drying, the ecosystem can undergo a state shift, turning the mire into a barren land with lower biodiversity and richness. Humic acid formation occurs during the biogeochemical degradation of vegetation debris and animal residue.[27] The loads of organic matter in the form of humic acid is a source of precursors of coal.[clarification needed] Prematurely exposing the organic matter to the atmosphere promotes the conversion of organics to carbon dioxide to be released in the atmosphere.
Use by humans
[edit]
Records of past human behaviour and environments can be contained within peatlands. These may take the form of human artefacts, or palaeoecological and geochemical records.[3]
Peatlands are used by humans in modern times for a range of purposes, the most dominant being agriculture and forestry, which accounts for around a quarter of global peatland area.[3] This involves cutting drainage ditches to lower the water table with the intended purpose of enhancing the productivity of forest cover or for use as pasture or cropland.[1] Agricultural uses for mires include the use of natural vegetation for hay crop or grazing, or the cultivation of crops on a modified surface.[2] In addition, the commercial extraction of peat for energy production is widely practiced in Northern European countries, such as Russia, Sweden, Finland, Ireland and the Baltic states.[3]
Tropical peatlands comprise 0.25% of Earth's terrestrial land surface but store 3% of all soil and forest carbon stocks.[28] The use of this land by humans, including draining and harvesting of tropical peat forests, results in the emission of large amounts of carbon dioxide into the atmosphere. In addition, fires occurring on peatland dried by the draining of peat bogs release even more carbon dioxide. The economic value of a tropical peatland was once derived from raw materials, such as wood, bark, resin and latex, the extraction of which did not contribute to large carbon emissions. In Southeast Asia, peatlands are drained and cleared for human use for a variety of reasons, including the production of palm oil and timber for export in primarily developing nations.[11] This releases stored carbon dioxide and preventing the system from sequestering carbon again.
Tropical peatlands
[edit]The global distribution of tropical peatlands is concentrated in Southeast Asia where agricultural use of peatlands has been increased in recent decades. Large areas of tropical peatland have been cleared and drained for the production of food and cash crops such as palm oil. Large-scale drainage of these plantations often results in subsidence, flooding, fire and deterioration of soil quality. Small scale encroachment on the other hand, is linked to poverty and is so widespread that it also has negatively impacts these peatlands.
The biotic and abiotic factors controlling Southeast Asian peatlands are interdependent.[2] Its soil, hydrology and morphology are created by the present vegetation through the accumulation of its own organic matter, building a favorable environment for this specific vegetation. This system is therefore vulnerable to changes in hydrology or vegetation cover.[29] These peatlands are mostly located in developing regions with impoverished and rapidly growing populations. These lands have become targets for commercial logging, paper pulp production and conversion to plantations through clear-cutting, drainage and burning.[2] Drainage of tropical peatlands alters the hydrology and increases their susceptibility to fire and soil erosion, as a consequence of changes in physical and chemical compositions.[30] The change in soil strongly affects the sensitive vegetation and forest die-off is common. The short-term effect is a decrease in biodiversity but the long-term effect, since these encroachments are hard to reverse, is a loss of habitat. Poor knowledge about peatlands' sensitive hydrology and lack of nutrients often lead to failing plantations, resulting in increasing pressure on remaining peatlands.[2]
Biology and peat characteristics
[edit]Tropical peatland vegetation varies with climate and location. Three different characterizations are mangrove woodlands present in the littoral zones and deltas of salty water, followed inland by swamp forests. These forests occur on the margin of peatlands with a palm rich flora with trees 70 m tall and 8 m in girth accompanied by ferns and epiphytes. The third, padang, from the Malay and Indonesian word for forest, consists of shrubs and tall thin trees and appear in the center of large peatlands.[2] The diversity of woody species, like trees and shrubs, are far greater in tropical peatlands than in peatlands of other types. Peat in the tropics is therefore dominated by woody material from trunks of trees and shrubs and contain little to none of the sphagnum moss that dominates in boreal peatlands.[2] It's only partly decomposed and the surface consists of a thick layer of leaf litter.[2] Forestry in peatlands leads to drainage and rapid carbon losses since it decreases inputs of organic matter and accelerate the decomposition.[31] In contrast to temperate wetlands, tropical peatlands are home to several species of fish. Many new, often endemic, species has been discovered but many of them are considered threatened.[30][32]
Greenhouse gases and fires
[edit]
The tropical peatlands in Southeast Asia only cover 0.2% of Earth's land area but CO2 emissions are estimated to be 2 Gt per year, equal to 7% of global fossil fuel emissions.[29] These emissions get bigger with drainage and burning of peatlands and a severe fire can release up to 4,000 t of CO2/ha. Burning events in tropical peatlands are becoming more frequent due to large-scale drainage and land clearance and in the past ten years, more than 2 million hectares was burnt in Southeast Asia alone. These fires last typically for 1–3 months and release large amounts of CO2.
Indonesia is one of the countries suffering from peatland fires, especially during years with ENSO-related drought, an increasing problem since 1982 as a result of developing land use and agriculture.[30] During the El Niño-event in 1997–1998 more than 24,400 km2[2] of peatland was lost to fires in Indonesia alone from which 10,000 km2 was burnt in Kalimantan and Sumatra. The output of CO2 was estimated to 0.81–2.57 Gt, equal to 13–40% of that year's global output from fossil fuel burning. Indonesia is now considered the third-biggest contributor to global CO2 emissions, caused primarily by these fires.[33] With a warming climate these burnings are expected to increase in intensity and number. This is a result of a dry climate together with an extensive rice farming project, called the Mega Rice Project, started in the 1990s, which converted 1 Mha of peatlands to rice paddies. Forest and land was cleared by burning and 4000 km of channels drained the area.[34] Drought and acidification of the lands led to bad harvest and the project was abandoned in 1999.[35] Similar projects in China have led to immense loss of tropical marshes and fens due to rice production.[36]
Drainage, which also increases the risk of burning, can cause additional emissions of CO2 by 30–100 t/ha/year if the water table is lowered by only 1 m.[37] The draining of peatlands is likely the most important and long-lasting threat to peatlands globally, but is especially prevalent in the tropics.[30]
Peatlands release the greenhouse gas methane which has strong global warming potential. However, subtropical wetlands have shown high CO2 binding per mol of released methane, which is a function that counteracts global warming.[38] Tropical peatlands are suggested to contain about 100 Gt carbon,[39][30] corresponding to more than 50% of the carbon present as CO2 in the atmosphere.[2] Accumulation rates of carbon during the last millennium were close to 40 g C/m2/yr.[40]
Northern peatlands
[edit]
Northern peatlands are associated with boreal and subarctic climates.[42] Northern peatlands were mostly built up during the Holocene after the retreat of Pleistocene glaciers, but in contrast tropical peatlands are much older. Total northern peat carbon stocks are estimated to be 1055 Gt of carbon.[43]
Of all northern circumpolar countries, Russia has the largest area of peatlands,[42] and contains the largest peatland in the world, The Great Vasyugan Mire.[44] Nakaikemi Wetland in southwest Honshu, Japan is more than 50,000 years old and has a depth of 45 m.[2] The Philippi Peatland in Greece has probably one of the deepest peat layers with a depth of 190 m.[45]
Impacts on global climate
[edit]According to the IPCC Sixth Assessment Report, the conservation and restoration of wetlands and peatlands has large economic potential to mitigate greenhouse gas emissions, providing benefits for adaptation, mitigation and biodiversity.[46]
Wetlands provide an environment where organic carbon is stored in living plants, dead plants and peat, as well as converted to carbon dioxide and methane. Three main factors give wetlands the ability to sequester and store carbon: high biological productivity, high water table and low decomposition rates. Suitable meteorological and hydrological conditions are necessary to provide an abundant water source for the wetland. Fully water-saturated wetland soils allow anaerobic conditions to manifest, storing carbon but releasing methane.[47]
Wetlands make up about 5-8% of Earth's terrestrial land surface but contain about 20-30% of the planet's 2500 Gt soil carbon stores.[48] Peatlands contain the highest amounts of soil organic carbon of all wetland types.[49] Wetlands can become sources of carbon, rather than sinks, as the decomposition occurring within the ecosystem emits methane.[47] Natural peatlands do not always have a measurable cooling effect on the climate in a short time span as the cooling effects of sequestering carbon are offset by the emission of methane, which is a strong greenhouse gas. However, given the short "lifetime" of methane (12 years), it is often said that methane emissions are unimportant within 300 years compared to carbon sequestration in wetlands. Within that time frame or less, most wetlands become both net carbon and radiative sinks. Hence, peatlands do result in cooling of the Earth's climate over a longer time period as methane is oxidised quickly and removed from the atmosphere whereas atmospheric carbon dioxide is continuously absorbed.[50] Throughout the Holocene (the past 12,000 years), peatlands have been persistent terrestrial carbon sinks and have had a net cooling effect, sequestering 5.6 to 38 grams of carbon per square metre per year. On average, it has been estimated that today northern peatlands sequester 20 to 30 grams of carbon per square metre per year.[1][51]
Peatlands insulate the permafrost in subarctic regions, thus delaying thawing during summer, as well as inducing the formation of permafrost.[50] As the global climate continues to warm, wetlands could become major carbon sources as higher temperatures cause higher carbon dioxide emissions.[52]
Compared with untilled cropland, wetlands can sequester around two times the carbon. Carbon sequestration can occur in constructed wetlands as well as natural ones. Estimates of greenhouse gas fluxes from wetlands indicate that natural wetlands have lower fluxes, but man-made wetlands have a greater carbon sequestration capacity. The carbon sequestration abilities of wetlands can be improved through restoration and protection strategies, but it takes several decades for these restored ecosystems to become comparable in carbon storage to peatlands and other forms of natural wetlands.[47]
Studies highlight the critical role of peatlands in biodiversity conservation and hydrological stability. These ecosystems are unique habitats for diverse species, including specific insects and amphibians, and act as natural water reservoirs, releasing water during dry periods to sustain nearby freshwater ecosystems and agriculture.[5]
Drainage for agriculture and forestry
[edit]The exchange of carbon between the peatlands and the atmosphere has been of current concern globally in the field of ecology and biogeochemical studies.[2] The drainage of peatlands for agriculture and forestry has resulted in the emission of extensive greenhouse gases into the atmosphere, most notably carbon dioxide and methane. By allowing oxygen to enter the peat column within a mire, drainage disrupts the balance between peat accumulation and decomposition, and the subsequent oxidative degradation results in the release of carbon into the atmosphere.[53] As such, drainage of mires for agriculture transforms them from net carbon sinks to net carbon emitters.[1] Although the emission of methane from mires has been observed to decrease following drainage,[20] the total magnitude of emissions from peatland drainage is often greater as rates of peat accumulation are low. Peatland carbon has been described as "irrecoverable" meaning that, if lost due to drainage, it could not be recovered within time scales relevant to climate mitigation.[54][55]
When undertaken in such a way that preserves the hydrological state of a mire, the anthropogenic use of mires' resources can avoid significant greenhouse gas emissions. However, continued drainage will result in increased release of carbon, contributing to global warming. As of 2016, it was estimated that drained peatlands account for around 10% of all greenhouse gas emissions from agriculture and forestry.[3]
Palm oil plantations
[edit]
Palm oil has increasingly become one of the world's largest crops. In comparison to alternatives, palm oil is considered to be among the most efficient sources of vegetable oil and biofuel, requiring only 0.26 hectares of land to produce 1 ton of oil.[56] Palm oil has therefore become a popular cash crop in many low-income countries and has provided economic opportunities for communities. With palm oil as a leading export in countries such as Indonesia and Malaysia, many smallholders have found economic success in palm oil plantations. However, the land selected for plantations are typically substantial carbon stores that promote biodiverse ecosystems.[57]
Palm oil plantations have replaced much of the forested peatlands in Southeast Asia. Estimates now state that 12.9 Mha or about 47% of peatlands in Southeast Asia were deforested by 2006.[58] In their natural state, peatlands are waterlogged with high water tables making for an inefficient soil.[clarification needed][56] To create viable soil for plantation, the mires in tropical regions of Indonesia and Malaysia are drained and cleared.
The peatland forests harvested for palm oil production serve as above- and below-ground carbon stores, containing at least 42,069 million metric tonnes (Mt) of soil carbon.[58] Exploitation of this land raises many environmental concerns, namely increased greenhouse gas emissions, risk of fires and a decrease in biodiversity. Greenhouse gas emissions for palm oil planted on peatlands is estimated to be between the equivalent of 12.4 (best case) to 76.6 t CO2/ha (worst case).[56] Tropical peatland converted to palm oil plantation can remain a net source of carbon to the atmosphere after 12 years.[59]
In their natural state, peatlands are resistant to fire. Drainage of peatlands for palm oil plantations creates a dry layer of flammable peat. As peat is carbon dense, fires occurring in compromised peatlands release extreme amounts of both carbon dioxide and toxic smoke into the air. These fires add to greenhouse gas emissions while also causing thousands of deaths every year.[citation needed]
Decreased biodiversity due to deforestation and drainage makes these ecosystem more vulnerable and less resilient to change. Homogenous ecosystems are at an increased risk to extreme climate conditions and are less likely to recover from fires.

Fires
[edit]Some peatlands are being dried out by climate change.[60] Drainage of peatlands due to climatic factors may also increase the risk of fires, presenting further risk of carbon and methane to release into the atmosphere.[3] Due to their naturally high moisture content, pristine mires have a generally low risk of fire ignition. The drying of this waterlogged state means that the carbon-dense vegetation becomes vulnerable to fire. In addition, due to the oxygen deficient nature of the vegetation, the peat fires can smolder beneath the surface causing incomplete combustion of the organic matter and resulting in extreme emissions events.[3]
In recent years, the occurrence of wildfires in peatlands has increased significantly worldwide particularly in the tropical regions. This can be attributed to a combination of drier weather and changes in land use which involve the drainage of water from the landscape.[1] This resulting loss of biomass through combustion has led to significant emissions of greenhouse gasses both in tropical and boreal/temperate peatlands.[61] Fire events are predicted to become more frequent with the warming and drying of the global climate.[2]
Management and rehabilitation
[edit]The United Nations Convention on Biological Diversity highlights peatlands as key ecosystems to be conserved and protected. The convention requires governments at all levels to present action plans for the conservation and management of wetland environments. Wetlands are also protected under the 1971 Ramsar Convention.[3]
Often, restoration is done by blocking drainage channels in the peatland, and allowing natural vegetation to recover.[62] Rehabilitation projects undertaken in North America and Europe usually focus on the rewetting of peatlands and revegetation of native species. This acts to mitigate carbon release in the short term before the new growth of vegetation provides a new source of organic litter to fuel the peat formation in the long term.[3] UNEP is supporting peatland restoration in Indonesia.[63]
Peat extraction is forbidden in Chile since April 2024.[64]
Global Peatlands Initiative
[edit]The Global Peatlands Initiative is an effort made by leading experts and institutions formed in 2016 by 13 founding members at the UNFCCC COP in Marrakech, Morocco.[65] The mission of the Initiative is to protect and conserve peatlands as the world's largest terrestrial organic carbon stock and to prevent it from being emitted into the atmosphere.
Members of the Initiative are working together within their respective areas of expertise to improve the conservation, restoration and sustainable management of peatlands. The Initiative is therefore contributing to several Sustainable Development Goals (SDGs), by keeping carbon stocks in the ground (SDG 13), by avoiding health impacts associated with serious air pollution from burning drained peatlands (SDG 3), by protecting water-related ecosystems and facilitating improved water quality (SDG 6), and by ensuring conservation of ecosystems and threatened species, protecting life on land (SDG 15).[66]References
[edit]- ^ a b c d e f g Frolking, Steve; Talbot, Julie; Jones, Miriam C.; Treat, Claire C.; Kauffman, J. Boone; Tuittila, Eeva-Stiina; and Roulet, Nigel (December 2011). "Peatlands in the Earth's 21st century climate system". Environmental Reviews. 19 (NA): 371–396. doi:10.1139/a11-014. ISSN 1181-8700.
- ^ a b c d e f g h i j k l m n o p q r Rydin, Håkan; Jeglum, J. K. (2013). The Biology of Peatlands. Bennett, Keith D. (2nd ed.). Oxford: Oxford University Press. ISBN 978-0-19-960299-5. OCLC 840132559.
- ^ a b c d e f g h i j k l Page, S.E. and Baird, A.J. (November 2016). "Peatlands and Global Change: Response and Resilience". Annual Review of Environment and Resources. 41 (1): 35–57. doi:10.1146/annurev-environ-110615-085520. ISSN 1543-5938.
- ^ "Wetlands Types and Classifications". Retrieved 20 May 2019.
- ^ a b Struzik, Ed (2021-09-16). "Why Saving World's Peatlands Can Help Stabilize the Climate". Yale E360. Retrieved 2024-05-19.
- ^ a b IUCN (November 2021). "Peatlands and climate change". iucn.org. Retrieved 2023-10-10.
- ^ Craft, Christopher (2022). Creating and Restoring Wetlands From Theory to Practice (2nd ed.). Elsevier. ISBN 978-0-12-823981-0.
- ^ Geist, Helmut (2006). Our Earth's Changing Land: An Encyclopedia of Land-Use and Land-Cover Change. Vol. 2. Greenwood. p. 463. ISBN 978-0-313-32784-1.
- ^ Rode, Elve (1999). "Wetland Restoration: A Survey of Options for Restoring Peatlands" (PDF). Studia Forestalia Suecica (205). Swedish University of Agricultural Sciences. ISBN 91-576-5557-X. ISSN 0039-3150. S2CID 199566835.
- ^ Gorham, Eville (1857). "The Development of Peat Lands". The Quarterly Review of Biology. 32 (2): 145–166. doi:10.1086/401755. S2CID 129085635.
- ^ a b c Page, Susan E.; Rieley, John O.; Banks, Christopher J. (2011-01-04). "Global and regional importance of the tropical peatland carbon pool" (PDF). Global Change Biology. 17 (2): 798–818. Bibcode:2011GCBio..17..798P. doi:10.1111/j.1365-2486.2010.02279.x. ISSN 1354-1013. S2CID 86121682.
- ^ "Restoring Indonesian peatlands, protecting our planet". UNOPS. Retrieved 2024-09-15.
- ^ Dargie, Greta C.; Lewis, Simon L.; Lawson, Ian T.; Mitchard, Edward T. A.; Page, Susan E.; Bocko, Yannick E.; Ifo, Suspense A. (2017-01-11). "Age, extent and carbon storage of the central Congo Basin peatland complex" (PDF). Nature (journal). 542 (7639): 86–90. Bibcode:2017Natur.542...86D. doi:10.1038/nature21048. ISSN 0028-0836. PMID 28077869. S2CID 205253362.
- ^ Joosten, H.; Clarke, D. (2002). Wise use of mires and peatlands. International Mire Conservation Group and International Peat Society.
- ^ Sandy, John H. "An Author Catalog of the Peat Research Collection at the University of Minnesota Duluth". Retrieved 2023-10-29.
- ^ Rydin, Håkan; Jeglum, John (2006). The Biology of Peatlands (1st ed.). Oxford University Press.
- ^ Belyea, Lisa R.; Malmer, Nils (July 2004). "Carbon sequestration in peatland: patterns and mechanisms of response to climate change". Global Change Biology. 10 (7): 1043–1052. Bibcode:2004GCBio..10.1043B. doi:10.1111/j.1529-8817.2003.00783.x. S2CID 39994255.
- ^ Leng, Lee Yit; Ahmed, Osumanu Haruna; Jalloh, Mohamadu Boyie (2019-03-01). "Brief review on climate change and tropical peatlands". Geoscience Frontiers. Climate change impacts on environmental geosciences. 10 (2): 373–380. doi:10.1016/j.gsf.2017.12.018. ISSN 1674-9871.
- ^ "Northern Ireland's peatlands face 'toxic' nitrogen risk". BBC News. 2022-01-25. Retrieved 2022-01-25.
- ^ Frolking, Steve; Roulet, Nigel T. (25 April 2007). "Holocene radiative forcing impact of northern peatland carbon accumulation and methane emissions". Global Change Biology. 13 (5): 1079–1088. doi:10.1111/j.1365-2486.2007.01339.x. ISSN 1354-1013.
- ^ Brown, Alastair (2011-12-20). "Carbon storage: When peat dries". Nature Climate Change. 2 (1): 22. doi:10.1038/nclimate1360.
- ^ Turetsky, M. R.; Treat, C. C.; Waldrop, M. P.; Waddington, J. M.; Harden, J. W.; McGuire, A. D. (2008-09-01). "Short-term response of methane fluxes and methanogen activity to water table and soil warming manipulations in an Alaskan peatland". Journal of Geophysical Research. 113 (G3): G00A10. Bibcode:2008JGRG..113.0A10T. doi:10.1029/2007jg000496. ISSN 2156-2202. S2CID 18756489.
- ^ Tobolski, K (2000). Przewodnik do oznaczania torfów i osadów jeziornych. PWN.
- ^ Kuske, E; Silamikele, Inese; Kalnina, Laimdota; Klavins, Maris (2010-01-01). "Peat formation conditions and peat properties: A study of two ombrotrophic bogs in Latvia". Mires and Peat.
- ^ Szajdak, L.W.; Szatylowicz, Jan (2010). "Impact of drainage on hydrophobicity of fen peat-moor soils". In M. Klavins (ed.). Mires and Peat. University of Latvia Press. pp. 158–174.
- ^ Chemistry, Gierlach-Hladon, T., Karol Marcinkowski Univ. of Medical Sciences, Poznan (Poland). Dept. of Inorganic and Analytical; Environment, Szajdak, L., Polish Academy of Sciences, Poznan (Poland). Inst. for Agricultural and Forest (2010). "Physico-chemical properties of humic acids isolated from an Eriophorum-Sphagnum raised bog". AGRIS: International Information System for the Agricultural Science and Technology. University of Latvia Press. ISBN 9789984451633.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "Carbon sequestration in peat bogs as a source of income". WUR. Archived from the original on 2018-04-09. Retrieved 2018-04-09.
- ^ a b Hooijer, A., Silvius, M., Wösten, H. and Page, S. 2006. PEAT-CO2, Assessment of CO2 emissions from drained peatlands in SE Asia. Delft Hydraulics report Q3943. [1]
- ^ a b c d e United Nations Environment Programme. Global Environment Facility. Asia Pacific Network for Global Change Research. Global Environment Centre (Malaysia), publisher. Wetlands International, publisher. (2008). Assessment on peatlands, biodiversity, and climate change. Global Environment Centre. ISBN 9789834375102. OCLC 933580381.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Biodiversity and sustainability of tropical peatlands : proceedings of the International Symposium on Biodiversity, Environmental Importance and Sustainability of Tropical Peat and Peatlands, held in Palangka Raya, Central Kalimantan, Indonesia, 4-8 September 1995. Rieley, Jack, 1941–, Page, Susan, 1957–. Cardigan, UK: Samara Pub. 1997. ISBN 1-873692-10-2. OCLC 37815652.
{{cite book}}: CS1 maint: others (link) - ^ Ng, Peter K. L.; Tay, J. B.; Lim, Kelvin K. P. (1994), "Diversity and conservation of blackwater fishes in Peninsular Malaysia, particularly in the North Selangor peat swamp forest", Ecology and Conservation of Southeast Asian Marine and Freshwater Environments including Wetlands, Springer Netherlands, pp. 203–218, doi:10.1007/978-94-011-0958-1_20, ISBN 9789401044141
- ^ Silvius, M., Kaat, A.H., Van de Bund and Hooijer, A. 2006. Peatland degradation fuels climate change. An unrecognised and alarming source of greenhouse gases. Wetlands International, Wageningen, The Netherlands.[2]
- ^ Boehm, H.-D. V., Siegert, F., Rieley, J. O. et al (2001). Fire impacts and carbon release on tropical peatlands in central Kalimantan, Indonesia. 22nd Asian Conference on Remote Sensing, 5–9 November 2001, Singapore. Centre for Remote Imaging, Sensing and Processing (CRISP), University of Singapore. [3]
- ^ Page, Susan; Hoscilo, Agata; Langner, Andreas; Tansey, Kevin; Siegert, Florian; Limin, Suwido; Rieley, Jack (2009), "Tropical peatland fires in Southeast Asia", Tropical Fire Ecology, Springer Berlin Heidelberg, pp. 263–287, doi:10.1007/978-3-540-77381-8_9, ISBN 978-3-540-77380-1
- ^ "'94 International Conference on Wetland Environment and Peatland Utilization". Chinese Geographical Science. 4 (1): 95. March 1994. doi:10.1007/bf02664953. ISSN 1002-0063. S2CID 195212972.
- ^ Wösten, J. H. M.; Van Den Berg, J.; Van Eijk, P.; Gevers, G. J. M.; Giesen, W. B. J. T.; Hooijer, A.; Idris, Aswandi; Leenman, P. H.; Rais, Dipa Satriadi (March 2006). "Interrelationships between Hydrology and Ecology in Fire Degraded Tropical Peat Swamp Forests". International Journal of Water Resources Development. 22 (1): 157–174. doi:10.1080/07900620500405973. ISSN 0790-0627. S2CID 154223494.
- ^ WHITING, GARY J.; CHANTON, JEFFREY P. (November 2001). "Greenhouse carbon balance of wetlands: methane emission versus carbon sequestration". Tellus B. 53 (5): 521–528. Bibcode:2001TellB..53..521W. doi:10.1034/j.1600-0889.2001.530501.x. ISSN 0280-6509.
- ^ Peatlands and climate change. Strack, Maria., International Peat Society. Jyväskylä, Finland: IPS, International Peat Society. 2008. ISBN 9789529940110. OCLC 404026180.
{{cite book}}: CS1 maint: others (link) - ^ Yu, Zicheng; Loisel, Julie; Brosseau, Daniel P.; Beilman, David W.; Hunt, Stephanie J. (July 2010). "Global peatland dynamics since the Last Glacial Maximum". Geophysical Research Letters. 37 (13): n/a. Bibcode:2010GeoRL..3713402Y. doi:10.1029/2010gl043584. ISSN 0094-8276.
- ^ Joosten H.; Tanneberger F.; Moen, A., eds. (2017). Mires and Peatlands of Europe. Schweizerbart Science Publishers. Stuttgart.
- ^ a b Tarnocai, C.; Stolbovoy, V. (2006). "Chapter 2 Northern Peatlands: their characteristics, development and sensitivity to climate change". In Martini, I. P.; Martínez Cortizas, A.; Chesworth, W. (eds.). Developments in Earth Surface Processes. Vol. 9. Elsevier. pp. 17–51. doi:10.1016/s0928-2025(06)09002-x. ISSN 0928-2025. Retrieved 2024-10-17.
- ^ Nichols, Jonathan E.; Peteet, Dorothy M. (21 October 2019). "Rapid expansion of northern peatlands and doubled estimate of carbon storage". Nature Geoscience. 12 (11): 917–921. Bibcode:2019NatGe..12..917N. doi:10.1038/s41561-019-0454-z. ISSN 1752-0908. S2CID 204812279.
- ^ Kirpotin, Sergey N.; Antoshkina, Olga A.; Berezin, Alexandr E.; Elshehawi, Samer; Feurdean, Angelica; Lapshina, Elena D.; Pokrovsky, Oleg S.; Peregon, Anna M.; Semenova, Natalia M.; Tanneberger, Franziska; Volkov, Igor V.; Volkova, Irina I.; Joosten, Hans (2021-11-01). "Great Vasyugan Mire: How the world's largest peatland helps addressing the world's largest problems". Ambio. 50 (11): 2038–2049. doi:10.1007/s13280-021-01520-2. ISSN 1654-7209. PMC 8497674. PMID 33677811.
- ^ Christanis, Kimon (2016). "The Philippi Peatland (Greece)". In Finlayson, C. Max; Milton, G. Randy; Prentice, R. Crawford; Davidson, Nick C. (eds.). The Wetland Book: II: Distribution, Description and Conservation. Springer Netherlands. pp. 1–6. doi:10.1007/978-94-007-6173-5_147-1. ISBN 9789400761735.
- ^ IPCC (2022). "Summary for Policymakers" (PDF). Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Archived from the original (PDF) on 2022-08-07. Retrieved 2022-05-20.
- ^ a b c Kayranli, Birol; Scholz, Miklas; Mustafa, Atif; Hedmark, Åsa (2010-02-01). "Carbon Storage and Fluxes within Freshwater Wetlands: a Critical Review". Wetlands. 30 (1): 111–124. doi:10.1007/s13157-009-0003-4. ISSN 0277-5212. S2CID 25306339.
- ^ Mitsch, William J.; Bernal, Blanca; Nahlik, Amanda M.; Mander, Ülo; Zhang, Li; Anderson, Christopher J.; Jørgensen, Sven E.; Brix, Hans (2013-04-01). "Wetlands, carbon, and climate change". Landscape Ecology. 28 (4): 583–597. doi:10.1007/s10980-012-9758-8. ISSN 0921-2973. S2CID 11939685.
- ^ Köchy, M.; Hiederer, R.; Freibauer, A. (2015-04-16). "Global distribution of soil organic carbon – Part 1: Masses and frequency distributions of SOC stocks for the tropics, permafrost regions, wetlands, and the world". Soil. 1 (1): 351–365. Bibcode:2015SOIL....1..351K. doi:10.5194/soil-1-351-2015. ISSN 2199-3971.
- ^ a b "Peatlands, climate change mitigation and biodiversity conservation | Ramsar". ramsar.org. Retrieved 2018-04-09.
- ^ Yu, Zicheng; Beilman, D. W.; Frolking, S.; MacDonald, G. M.; Roulet, N. T.; Camill, P.; Charman, D. J. (2011). "Peatlands and Their Role in the Global Carbon Cycle". Eos, Transactions American Geophysical Union. 92 (12): 97–98. Bibcode:2011EOSTr..92...97Y. doi:10.1029/2011EO120001. ISSN 2324-9250.
- ^ Turetsky, Merritt R.; Abbott, Benjamin W.; Jones, Miriam C.; Walter Anthony, Katey; Olefeldt, David; Schuur, Edward A. G.; Koven, Charles; McGuire, A. David; Grosse, Guido (2019-04-30). "Permafrost collapse is accelerating carbon release". Nature (journal). 569 (7754): 32–34. Bibcode:2019Natur.569...32T. doi:10.1038/d41586-019-01313-4. ISSN 0028-0836. PMID 31040419.
- ^ Minkkinen, Kari; Laine, Jukka (1998). "Long-term effect of forest drainage on the peat carbon stores of pine mires in Finland". Canadian Journal of Forest Research. 28 (9): 1267–1275. doi:10.1139/x98-104.
- ^ Goldstein, Allie; Turner, Will R.; Spawn, Seth A.; Anderson-Teixeira, Kristina J.; Cook-Patton, Susan; Fargione, Joseph; Gibbs, Holly K.; Griscom, Bronson; Hewson, Jennifer H.; Howard, Jennifer F.; Ledezma, Juan Carlos; Page, Susan; Koh, Lian Pin; Rockström, Johan; Sanderman, Jonathan; Hole, David G. (April 2020). "Protecting irrecoverable carbon in Earth's ecosystems". Nature Climate Change. 10 (4): 287–295. Bibcode:2020NatCC..10..287G. doi:10.1038/s41558-020-0738-8. S2CID 214718837.
- ^ Noon, Monica L.; Goldstein, Allie; Ledezma, Juan Carlos; Roehrdanz, Patrick R.; Cook-Patton, Susan C.; Spawn-Lee, Seth A.; Wright, Timothy Maxwell; Gonzalez-Roglich, Mariano; Hole, David G.; Rockström, Johan; Turner, Will R. (January 2022). "Mapping the irrecoverable carbon in Earth's ecosystems". Nature Sustainability. 5 (1): 37–46. doi:10.1038/s41893-021-00803-6. S2CID 244349665.
- ^ a b c d Hashim, Zulkifli; Subramaniam, Vijaya; Harun, Mohd Haniff; Kamarudin, Norman (June 2018). "Carbon footprint of oil palm planted on peat in Malaysia". The International Journal of Life Cycle Assessment. 23 (6): 1201–1217. doi:10.1007/s11367-017-1367-y. ISSN 0948-3349. S2CID 115328269.
- ^ Laurance, William F.; Koh, Lian P.; Butler, Rhett; Sodhi, Navjot S.; Bradshaw, Corey J. A.; Neidel, J. David; Consunji, Hazel; Mateo Vega, Javier (April 2010). "Improving the Performance of the Roundtable on Sustainable Palm Oil for Nature Conservation". Conservation Biology. 24 (2): 377–381. doi:10.1111/j.1523-1739.2010.01448.x. ISSN 0888-8892. PMID 20184655.
- ^ a b Hooijer, A.; Page, S.; Canadell, J. G.; Silvius, M.; Kwadijk, J.; Wösten, H.; Jauhiainen, J. (2010-05-12). "Current and future CO2 emissions from drained peatlands in Southeast Asia". Biogeosciences. 7 (5): 1505–1514. Bibcode:2010BGeo....7.1505H. doi:10.5194/bg-7-1505-2010. ISSN 1726-4189.
- ^ McCalmont, Jon; Kho, Lip Khoon; Teh, Yit Arn; Lewis, Kennedy; Chocholek, Melanie; Rumpang, Elisa; Hill, Timothy (2 February 2021). "Short- and long-term carbon emissions from oil palm plantations converted from logged tropical peat swamp forest". Global Change Biology. 27 (11): 2361–2376. Bibcode:2021GCBio..27.2361M. doi:10.1111/gcb.15544. hdl:2164/17863. ISSN 1354-1013. PMID 33528067. S2CID 231757053.
- ^ "Climate change threatening buried UK treasures". BBC News. 2022-01-25. Retrieved 2022-01-25.
- ^ Granath, Gustaf; Moore, Paul A.; Lukenbach, Maxwell C.; Waddington, James M. (2016-06-27). "Mitigating wildfire carbon loss in managed northern peatlands through restoration". Scientific Reports. 6 (1): 28498. Bibcode:2016NatSR...628498G. doi:10.1038/srep28498. ISSN 2045-2322. PMC 4921962. PMID 27346604.
- ^ "The natural world can help save us from climate catastrophe | George Monbiot". The Guardian. April 3, 2019.
- ^ Environment, U. N. (2020-08-10). "UNEP supports project to restore peatlands in Indonesia". UN Environment. Retrieved 2020-08-11.
- ^ "Ley 21660 sobre protección ambiental de las turberas". bcn.cl (in Spanish). Biblioteca del Congreso Nacional. 2024. Retrieved 2024-09-11.
- ^ "New UN initiative aims to save lives and cut climate change by protecting peatlands - United Nations Sustainable Development". United Nations Sustainable Development. 2016-11-17. Retrieved 2017-12-16.
- ^ "Carbon, biodiversity and land-use in the Central Congo Basin Peatlands".
External links
[edit]- . Encyclopædia Britannica. Vol. 22 (11th ed.). 1911. p. 703.
