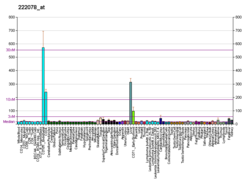
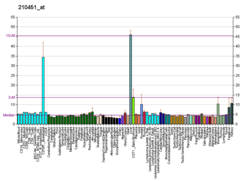
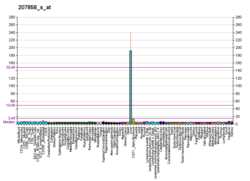
Pyruvate kinase PKLR
Appearance
(Redirected from PKLR)
Pyruvate kinase PKLR is an enzyme that in humans is encoded by the PKLR gene.[5][6]
The protein encoded by this gene is a pyruvate kinase that catalyzes the production of pyruvate and ATP from phosphoenolpyruvate. Defects in this enzyme, due to gene mutations or genetic variations, are the common cause of chronic hereditary nonspherocytic hemolytic anemia (CNSHA or HNSHA). Alternatively spliced transcript variants encoding distinct isoforms have been described.[6]
Interactive pathway map
[edit]Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
Glycolysis and Gluconeogenesis edit
- ^ The interactive pathway map can be edited at WikiPathways: "GlycolysisGluconeogenesis_WP534".
References
[edit]- ^ a b c ENSG00000143627 GRCh38: Ensembl release 89: ENSG00000262785, ENSG00000143627 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000041237 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "PKLR - Pyruvate kinase PKLR - Homo sapiens (Human) - PKLR gene & protein". www.uniprot.org. Retrieved 26 March 2022.
- ^ a b "Entrez Gene: PKLR pyruvate kinase, liver and RBC".
Further reading
[edit]- Beutler E, Baronciani L (1996). "Mutations in pyruvate kinase". Hum. Mutat. 7 (1): 1–6. doi:10.1002/(SICI)1098-1004(1996)7:1<1::AID-HUMU1>3.0.CO;2-H. PMID 8664896. S2CID 2481467.
- Baronciani L, Bianchi P, Zanella A (1999). "Hematologically important mutations: red cell pyruvate kinase (2nd update)". Blood Cells Mol. Dis. 24 (3): 273–9. doi:10.1006/bcmd.1998.0193. PMID 10087985.
- Zanella A, Fermo E, Bianchi P, et al. (2007). "Pyruvate kinase deficiency: the genotype-phenotype association". Blood Rev. 21 (4): 217–31. doi:10.1016/j.blre.2007.01.001. PMID 17360088.
- Kanno H, Fujii H, Miwa S (1992). "Structural analysis of human pyruvate kinase L-gene and identification of the promoter activity in erythroid cells". Biochem. Biophys. Res. Commun. 188 (2): 516–23. doi:10.1016/0006-291X(92)91086-6. PMID 1445295.
- Kanno H, Fujii H, Hirono A, et al. (1992). "Identical point mutations of the R-type pyruvate kinase (PK) cDNA found in unrelated PK variants associated with hereditary hemolytic anemia". Blood. 79 (5): 1347–50. doi:10.1182/blood.V79.5.1347.1347. PMID 1536957.
- Dawson SJ, White LA (1992). "Treatment of Haemophilus aphrophilus endocarditis with ciprofloxacin". J. Infect. 24 (3): 317–20. doi:10.1016/S0163-4453(05)80037-4. PMID 1602151.
- Kanno H, Fujii H, Hirono A, Miwa S (1991). "cDNA cloning of human R-type pyruvate kinase and identification of a single amino acid substitution (Thr384----Met) affecting enzymatic stability in a pyruvate kinase variant (PK Tokyo) associated with hereditary hemolytic anemia". Proc. Natl. Acad. Sci. U.S.A. 88 (18): 8218–21. Bibcode:1991PNAS...88.8218K. doi:10.1073/pnas.88.18.8218. PMC 52478. PMID 1896471.
- Neubauer B, Lakomek M, Winkler H, et al. (1991). "Point mutations in the L-type pyruvate kinase gene of two children with hemolytic anemia caused by pyruvate kinase deficiency". Blood. 77 (9): 1871–5. doi:10.1182/blood.V77.9.1871.1871. PMID 2018831.
- Tani K, Fujii H, Nagata S, Miwa S (1988). "Human liver type pyruvate kinase: complete amino acid sequence and the expression in mammalian cells". Proc. Natl. Acad. Sci. U.S.A. 85 (6): 1792–5. Bibcode:1988PNAS...85.1792T. doi:10.1073/pnas.85.6.1792. PMC 279865. PMID 3126495.
- Satoh H, Tani K, Yoshida MC, et al. (1988). "The human liver-type pyruvate kinase (PKL) gene is on chromosome 1 at band q21". Cytogenet. Cell Genet. 47 (3): 132–3. doi:10.1159/000132530. PMID 3378452.
- Baronciani L, Beutler E (1995). "Molecular study of pyruvate kinase deficient patients with hereditary nonspherocytic hemolytic anemia". J. Clin. Invest. 95 (4): 1702–9. doi:10.1172/JCI117846. PMC 295683. PMID 7706479.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Kanno H, Ballas SK, Miwa S, et al. (1994). "Molecular abnormality of erythrocyte pyruvate kinase deficiency in the Amish". Blood. 83 (8): 2311–6. doi:10.1182/blood.V83.8.2311.2311. PMID 8161798.
- Lenzner C, Nürnberg P, Thiele BJ, et al. (1994). "Mutations in the pyruvate kinase L gene in patients with hereditary hemolytic anemia". Blood. 83 (10): 2817–22. doi:10.1182/blood.V83.10.2817.2817. PMID 8180378.
- Kanno H, Fujii H, Tsujino G, Miwa S (1993). "Molecular basis of impaired pyruvate kinase isozyme conversion in erythroid cells: a single amino acid substitution near the active site and decreased mRNA content of the R-type PK". Biochem. Biophys. Res. Commun. 192 (1): 46–52. doi:10.1006/bbrc.1993.1379. PMID 8476433.
- Kanno H, Fujii H, Miwa S (1993). "Low substrate affinity of pyruvate kinase variant (PK Sapporo) caused by a single amino acid substitution (426 Arg-->Gln) associated with hereditary hemolytic anemia". Blood. 81 (9): 2439–41. doi:10.1182/blood.V81.9.2439.bloodjournal8192439. PMID 8481523.
- Baronciani L, Beutler E (1993). "Analysis of pyruvate kinase-deficiency mutations that produce nonspherocytic hemolytic anemia". Proc. Natl. Acad. Sci. U.S.A. 90 (9): 4324–7. Bibcode:1993PNAS...90.4324B. doi:10.1073/pnas.90.9.4324. PMC 46499. PMID 8483951.
- Baronciani L, Bianchi P, Zanella A (1996). "Hematologically important mutations: red cell pyruvate kinase". Blood Cells Mol. Dis. 22 (1): 85–9. doi:10.1006/bcmd.1996.0012. PMID 8807089.