Aquatic plant


Aquatic plants also referred to as hydrophytes[1] are vascular plants and non-vascular plants[2] that have adapted to live in aquatic environments (saltwater or freshwater). In lakes, rivers and wetlands, aquatic vegetations provide cover for aquatic animals such as fish, amphibians and aquatic insects, create substrate for benthic invertebrates, produce oxygen via photosynthesis, and serve as food for some herbivorous wildlife.[3] Familiar examples of aquatic plants include waterlily, lotus, duckweeds, mosquito fern, floating heart, water milfoils, mare's tail, water lettuce, water hyacinth, and algae.[4]
Aquatic plants require special adaptations for prolonged inundation in water, and for floating at the water surface. The most common adaptation is the presence of lightweight internal packing cells, aerenchyma, but floating leaves and finely dissected leaves are also common.[5][6][7] Aquatic plants only thrive in water or in soil that is frequently saturated, and are therefore a common component of swamps and marshlands.[8]
Evolution
[edit]Aquatic plants have adapted to live in either freshwater or saltwater. Aquatic vascular plants have originated on multiple occasions in different plant families;[5][9] they can be ferns or angiosperms (including both monocots and dicots). The only angiosperms capable of growing completely submerged in seawater are the seagrasses.[10] Examples are found in genera such as Thalassia and Zostera. An aquatic origin of angiosperms is supported by the evidence that several of the earliest known fossil angiosperms were aquatic. Aquatic plants are phylogenetically well dispersed across the angiosperms, with at least 50 independent origins, although they comprise less than 2% of the angiosperm species.[11] Archaefructus represents one of the oldest, most complete angiosperm fossils which is around 125 million years old.[12] These plants require special adaptations for living submerged in water or floating at the surface.[12]
Morphology
[edit]Fully submerged aquatic plants have little need for stiff or woody tissue as they are able to maintain their position in the water using buoyancy typically from gas filled lacunaa or turgid Aerenchyma cells.[13] When removed from the water, such plants are typically limp and lose turgor rapidly.[14]
Those living in rivers do, however, need sufficient structural xylem to avoid being damaged by fast flowing water and they also need strong mechanisms of attachment to avoid being uprooted by river flow.
Many fully submerged plants have finely dissected leaves, probably to reduce drag in rivers and to provide a much increased surface area for interchange of minerals and gasses.[13] Some species of plants such as Ranunculus aquatilis have two different leaf forms with finely dissected leaves that are fully submerged and entire leaves on the surface of the water.
Some still-water plants can alter their position in the water column at different seasons. One notable example is Water soldier which rests as a rootless rosette on the bottom of the water body but slowly floats to the surface in late Spring so that its inflorescence can emerge into the air. While it is ascending through the water column it produces roots and vegetative daughter plants by means of rhizomes. When flowering is complete, the plant descends through the water column and the roots atrophy.
In floating aquatic angiosperms, the leaves have evolved to only have stomata on the top surface to make use of atmospheric carbon dioxide.[15] Gas exchange primarily occurs through the top surface of the leaf due to the position of the stomata, and the stomata are in a permanently open state. Due to their aquatic surroundings, the plants are not at risk of losing water through the stomata and therefore face no risk of dehydration.[15] For carbon fixation, some aquatic angiosperms are able to uptake CO2 from bicarbonate in the water, a trait that does not exist in terrestrial plants.[16] Angiosperms that use HCO
3- can keep CO2 levels satisfactory, even in basic environments with low carbon levels.[16] The many possible classifications of aquatic plants are based upon morphology.[5]
Classification
[edit]Aquatic plants are either aquatic macrophytes or aquatic microphytes.[17] Aquatic macrophytes are hydrophytes that are large enough to be seen with the naked eye.[18] Aquatic microphytes are hydrophytes that cannot be seen with the naked eye; they are microscopic.[17]
Classification of Macrophytes:
[edit]Based on growth form, macrophytes can be characterized as:[19]
- Emergent
- Submerged
- Rooted: rooted to the substrate
- Unrooted: free-floating in the water column
- Floating-leaved
- Free-floating
Emergent:
[edit]- An emergent plant is one which grows in water but pierces the surface so that it is partially exposed to air. Collectively, such plants are emergent vegetation.[20]
- This habit may have developed because the leaves can photosynthesize more efficiently in air and competition from submerged plants but often, the main aerial feature is the flower and the related reproductive process. The emergent habit permits pollination by wind or by flying insects.[20][21]
- There are many species of emergent plants, among them, the reed (Phragmites), Cyperus papyrus, Typha species, flowering rush and wild rice species. Some species, such as purple loosestrife, may grow in water as emergent plants but they are capable of flourishing in fens or simply in damp ground.[22]
Submergent:
[edit]- Submerged macrophytes completely grow under water with roots attached to the substrate (rooted submerged) (e.g. Myriophyllum spicatum) or without any root system (unrooted submerged) (e.g. Ceratophyllum demersum). They can also grow up to the water's surface.[23] Helophytes are plants that grow partly submerged in marshes and regrow from buds below the water surface.[24] Fringing stands of tall vegetation by water basins and rivers may include helophytes. Examples include stands of Equisetum fluviatile, Glyceria maxima, Hippuris vulgaris, Sagittaria, Carex, Schoenoplectus, Sparganium, Acorus, yellow flag (Iris pseudacorus), Typha and Phragmites australis.[24]
- Although seaweeds, which are large multicellular marine algae, have similar ecological functions to aquatic plants such as seagrass, they lack the specialized root/rhizoid system of plants.[25] Instead, seaweeds have holdfasts that only serve as anchors and have no absorptive functions.[26]
Floating-leaved:
[edit]- Floating-leaved macrophytes have root systems attached to the substrate or bottom of the body of water and with leaves that float on the water surface. Common floating leaved macrophytes are water lilies (family Nymphaeaceae), pondweeds (family Potamogetonaceae).[27]
Free-floating:
[edit]- floating freely on the water's surface.[28]
- Free-floating macrophytes are found suspended on water surface with their root not attached to the substrate, sediment, or bottom of the water body. They are easily blown by air and provide breeding ground for mosquitoes. Examples include Pistia spp. commonly called water lettuce, water cabbage or Nile cabbage.[27]
Classification of Microphytes:
[edit]Microphytes can be characterized as:[19][29]
- Phytoplankton
- Periphyton
Phytoplankton:
[edit]Periphyton:
[edit]- a microphyte that lives and grows on the surface of rooted aquatic plants. [31]
Additional Morphological Classifications:
[edit]The many possible classifications of aquatic plants are based upon morphology.[5] One example has six groups as follows:[32]
- Amphiphytes: plants that are adapted to live either submerged or on land
- Elodeids: stem plants that complete their entire lifecycle submerged, or with only their flowers above the waterline
- Isoetids: rosette plants that complete their entire lifecycle submerged
- Helophytes: plants rooted in the bottom, but with leaves above the waterline
- Nymphaeids: plants rooted in the bottom, but with leaves floating on the water surface
- Neuston: vascular plants that float freely in the water
Aquatic Adaptations
[edit]Terrestrial plants in aquatic environments
[edit]Terrestrial plants may undergo physiological changes when submerged due to flooding. When submerged, new leaf growth has been found to have thinner leaves and thinner cell walls than the leaves on the plant that grew while above water, along with oxygen levels being higher in the portion of the plant grown underwater versus the sections that grew in their terrestrial environment.[33] This is considered a form of phenotypic plasticity as the plant, once submerged, experiences changes in morphology better suited to their new aquatic environment.[33] However, while some terrestrial plants may be able to adapt in the short-term to an aquatic habitat, it may not be possible to reproduce underwater, especially if the plant usually relies on terrestrial pollinators.
Buoyancy
[edit]Due to their environment, aquatic plants experience buoyancy which counteracts their weight.[34] Because of this, their cell covering are far more flexible and soft, due to a lack of pressure that terrestrial plants experience.[34] Green algae are also known to have extremely thin cell walls due to their aquatic surroundings, and research has shown that green algae is the closest ancestor to living terrestrial and aquatic plants.[35] Terrestrial plants have rigid cell walls meant for withstanding harsh weather, as well as keeping the plant upright as the plant resists gravity. Gravitropism, along with phototropism and hydrotropism, are traits believed to have evolved during the transition from an aquatic to terrestrial habitat.[36][37] Terrestrial plants no longer had unlimited access to water and had to evolve to search for nutrients in their new surroundings as well as develop cells with new sensory functions, such as statocytes.
Photosynthesis
[edit]Submerged aquatic plants have more restricted access to carbon as carbon dioxide compared to terrestrial plants. They may also experience reduced light levels.[16] In aquatic plants diffuse boundary layers (DBLs) around submerged leaves and photosynthetic stems vary based on the leaves' thickness, shape and density and are the main factor responsible for the greatly reduced rate of gaseous transport across the leaf/water boundary and therefore greatly inhibit transport of carbon dioxide.[16] To overcome this limitation, many aquatic plants have evolved to metabolise bicarbonate ions as a source of carbon.[16]
Environmental variables affect the instantaneous photosynthetic rates of aquatic plants and the photosynthetic enzymes pigments.[38] In water, light intensity rapidly decreases with depth. Respiration is also higher in the dark per the unit volume of the medium they live in.[38]
Reproduction
[edit]Although most aquatic angiosperms can reproduce by flowering and setting seeds, many have also evolved to have extensive asexual reproduction by means of rhizomes, turions, and fragments in general.[6]
Functions of Aquatic Plants
[edit]One of the largest aquatic plants in the world is the Bolivian waterlily, which holds the Guinness World Record of having the largest undivided leaf at 3.2 m (10 ft 6 in) diameter; the smallest is the rootless duckweed, which is only 1 mm (0.039 in) across. Many small animals use aquatic plants such as duckweeds and lily pads for spawning or as protective shelters against predators both from above and below the water surface.
Aquatic plants are important primary producers and are the basis of food web for many aquatic fauna, especially wetland species.[39] They compete with phytoplanktons for excess nutrients such as nitrogen and phosphorus, thus reducing the prevalence of eutrophication and harmful algal blooms, and have a significant effect on riparian soil chemistry[40] as their leaves, stems and roots slow down the water flow, capture sediments and trap pollutants. Excess sediment will settle into the stream bed due to the reduced flow rates, and some aquatic plants also have symbiotic microbes capable of nitrogen fixation and breaking down the pollutants trapped and/or absorbed by the roots.[41][25] Historically, aquatic plants have been less studied than terrestrial plants,[42] and management of aquatic vegetation has become an increasingly interested field[43] as means to reduce agricultural pollution of water bodies.[44][45]
Functions of macrophytes in aquatic systems
[edit]Macrophytes perform many ecosystem functions in aquatic ecosystems and provide services to human society. One of the important functions performed by macrophyte is uptake of dissolved nutrients including nitrogen and phosphorus.[40] Macrophytes are widely used in constructed wetlands around the world to remove excess N and P from polluted water.[46] Beside direct nutrient uptake, macrophytes indirectly influence nutrient cycling, especially N cycling through influencing the denitrifying bacterial functional groups that are inhabiting on roots and shoots of macrophytes.[47] Macrophytes promote the sedimentation of suspended solids by reducing the current velocities,[48] impede erosion by stabilising soil surfaces.[49] Macrophytes also provide spatial heterogeneity in otherwise unstructured water column. Habitat complexity provided by macrophytes tends to increase diversity and density of both fish and invertebrates.[50]
The additional site-specific macrophytes' value provides wildlife habitat and makes treatment systems of wastewater aesthetically satisfactory.[51]
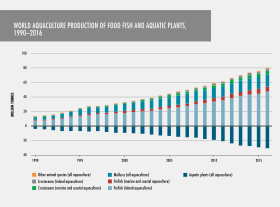
Uses and importance to humans
[edit]- Food crops
Some aquatic plants are used by humans as a food source. Examples include wild rice (Zizania), water caltrop (Trapa natans), Chinese water chestnut (Eleocharis dulcis), Indian lotus (Nelumbo nucifera), water spinach (Ipomoea aquatica), prickly waterlily (Euryale ferox), and watercress (Rorippa nasturtium-aquaticum).
- Bioassessment
A decline in a macrophyte community may indicate water quality problems and changes in the ecological status of the water body. Such problems may be the result of excessive turbidity, herbicides, or salination. Conversely, overly high nutrient levels may create an overabundance of macrophytes, which may in turn interfere with lake processing.[3] Macrophyte levels are easy to sample, do not require laboratory analysis, and are easily used for calculating simple abundance metrics.[3]
- Potential sources of therapeutic agents
Phytochemical and pharmacological researches suggest that freshwater macrophytes, such as Centella asiatica, Nelumbo nucifera, Nasturtium officinale, Ipomoea aquatica and Ludwigia adscendens, are promising sources of anticancer and antioxidative natural products.[52]
Hot water extracts of the stem and root of Ludwigia adscendens, as well as those of the fruit, leaf and stem of Monochoria hastata were found to have lipoxygenase inhibitory activity. Hot water extract prepared from the leaf of Ludwigia adscendens exhibits alpha-glucosidase inhibitory activity more potent than that of acarbose.[53]
- Wastewater treatment
Macrophytes have an essential role in some forms of wastewater treatment, most commonly in small scale sewage treatment using constructed wetlands or in polishing lagoons for larger schemes.[51]
Distribution
[edit]The principal factor controlling the distribution of aquatic plants is the availability of water. However, other abiotic factors may also control their distribution including nutrient availability, availability of carbon dioxide and oxygen, water temperature, characteristics of the substrate, water transparency,[54] water movement, and salinity.[8] Some aquatic plants are able to thrive in brackish, saline, and salt water.[5] Also biotic factors like grazing,[8] competition for light,[54] colonization by fungi,[55] and allelopathy[56] are influencing the occurrence of macrophytes.
Invasive aquatic plants
[edit]The introduction of non-native aquatic plants has resulted in numerous examples across the world of such plants becoming invasive and frequently dominating the environments into which they have been introduced.[57] Such species include Water hyacinth which is invasive in many tropical and sub-tropical locations including much of the southern US, many Asian countries and Australia. New Zealand stonecrop is a highly invasive plant in temperate climates spreading from a marginal plant to encompassing the whole body of many ponds to the almost total exclusion of other plants and wildlife[58]
Other notable invasive plant species include floating pennywort,[59] Curly leaved pondweed,[58] the fern ally Water fern[58] and Parrot's feather.[60] Many of these invasive plants have been sold as oxygenating plants for aquaria or decorative plants for garden ponds and have then been disposed of into the environment.[58]
In 2012, a comprehensive overview of alien aquatic plants in 46 European countries found 96 alien aquatic species. The aliens were primarily native to North America, Asia, and South America. The most spread alien plant in Europe was Elodea canadensis (Found in 41 European countries) followed by Azolla filiculoides in 25 countries and Vallisneria spiralis in 22 countries.[57] The countries with the most recorded alien aquatic plant species were France and Italy with 30 species followed by Germany with 27 species, and Belgium and Hungary with 26 species.[57]
The European and Mediterranean Plant Protection Organization has published recommendations to European nations advocating the restriction or banning of the trade in invasive alien plants.[61]
See also
[edit]- Aquatic animal
- Aquatic Botany (journal)
- Aquatic
- Aquatic ecosystem
- Aquatic locomotion
- Aquatic mammal
- Botany
- List of freshwater aquarium plant species
- List of wetland plants
- Marine biology
- Plant community
- Raunkiær plant life-form
- Terrestrial animal
- Terrestrial ecosystem
- Terrestrial locomotion
- Terrestrial plant
- Wetland – Type of land area that is flooded or saturated with water
- Wetland indicator status
References
[edit]- ^ "Dictionary.com | Meanings & Definitions of English Words". Dictionary.com. Retrieved 2024-12-11.
- ^ "What Are Aquatic Plants and Algae | manoa.hawaii.edu/ExploringOurFluidEarth". manoa.hawaii.edu. Retrieved 2024-12-11.
- ^ a b c "Macrophytes as Indicators of freshwater marshes in Florida" (PDF). Archived (PDF) from the original on 2014-04-07. Retrieved 2014-04-05.
- ^ "Aquatic Plants - Definition, Types, and Importance of Aquatic Plants". Toppr-guides. 2019-11-05. Retrieved 2024-12-11.
- ^ a b c d e Sculthorpe, C. D. 1967. The Biology of Aquatic Vascular Plants. Reprinted 1985 Edward Arnold, by London.
- ^ a b Hutchinson, G. E. 1975. A Treatise on Limnology, Vol. 3, Limnological Botany. New York: John Wiley.
- ^ Cook, C.D.K. (ed). 1974. Water Plants of the World. Dr W Junk Publishers, The Hague. ISBN 90-6193-024-3.
- ^ a b c Keddy, P.A. 2010. Wetland Ecology: Principles and Conservation (2nd edition). Cambridge University Press, Cambridge, UK. 497 p.
- ^ Tomlinson, P. B. (1986). The Botany of Mangroves. Cambridge, UK: Cambridge University Press.
- ^ "Alismatales". Angiosperm Phylogeny Website. Missouri Botanical Garden. Archived from the original on 2018-01-29. Retrieved 2018-03-01.
- ^ Pennisi, Elizabeth (2018-06-01). "This saltwater trout evolved to live in freshwater—in just 100 years". Science. doi:10.1126/science.aau3582. ISSN 0036-8075. S2CID 89661781.
- ^ a b Mader, Sylvia S. (1998). Biology. WCB/McGraw-Hill. ISBN 0-697-34079-1. OCLC 37418228.
- ^ a b "Morphological, Physiological and Anatomical Adaptations in Plants". Aligarh Muslim University. Retrieved 8 February 2022.
- ^ "Plant Adaptations to Aquatic Life". The Offwell Woodland & Wildlife Trust. Retrieved 8 February 2022.
- ^ a b Shtein, Ilana; Popper, Zoë A.; Harpaz-Saad, Smadar (2017-07-03). "Permanently open stomata of aquatic angiosperms display modified cellulose crystallinity patterns". Plant Signaling & Behavior. 12 (7): e1339858. Bibcode:2017PlSiB..12E9858S. doi:10.1080/15592324.2017.1339858. ISSN 1559-2324. PMC 5586356. PMID 28718691.
- ^ a b c d e Pedersen, Ole; Colmer, Timothy David; Sand-Jensen, Kaj (2013). "Underwater Photosynthesis of Submerged Plants – Recent Advances and Methods". Frontiers in Plant Science. 4: 140. doi:10.3389/fpls.2013.00140. ISSN 1664-462X. PMC 3659369. PMID 23734154.
- ^ a b Edo, GI; Nwosu, LC; Samuel, PO (2023). "Assessment of the physicochemical-water quality parameters and distribution of aquatic macrophytes present in Goenyeli Dam, Nicosia district, North Cyprus" (PDF). J Anal Pharm Res. 12 (1): 19–24.
- ^ "Dictionary.com | Meanings & Definitions of English Words". Dictionary.com. Retrieved 2024-12-11.
- ^ a b c "The decrease in aquatic vegetation in Europe and its consequences for fish populations - 2. plants in the aquatic habitat". www.fao.org. Retrieved 2024-12-11.
- ^ a b "What Are The Different Types Of Aquatic Plants?". WorldAtlas. 2019-10-08. Retrieved 2023-12-01.
- ^ Vymazal, Jan (December 2013). "Emergent plants used in free water surface constructed wetlands: A review". Ecological Engineering. 61: 582–592. Bibcode:2013EcEng..61..582V. doi:10.1016/j.ecoleng.2013.06.023. ISSN 0925-8574.
- ^ Swearingen, Jil M. (7 July 2009). "PCA Alien Plant Working Group – Purple Loosestrife (Lythrum salicaria)". National Park Service. Archived from the original on 2 September 2011. Retrieved 24 September 2011.
- ^ Bonsack, Kara (2016-10-20). "Submerged Aquatic Vegetation | Adapt CT". Retrieved 2024-12-11.
- ^ a b Beentje, Henk; Hickey, Michael; King, Clive (2001). "The Cambridge Illustrated Glossary of Botanical Terms". Kew Bulletin. 56 (2): 505. Bibcode:2001KewBu..56..505B. doi:10.2307/4110976. ISSN 0075-5974. JSTOR 4110976. S2CID 86620932.
- ^ a b Krause-Jensen, Dorte; Sand-Jensen, Kaj (May 1998). "Light attenuation and photosynthesis of aquatic plant communities". Limnology and Oceanography. 43 (3): 396–407. Bibcode:1998LimOc..43..396K. doi:10.4319/lo.1998.43.3.0396. ISSN 0024-3590. S2CID 85700950.
- ^ "22 Seaweed Facts to Know in 2022". Kvaroy Arctic. Retrieved 2024-12-11.
- ^ a b Bornette, Gudrun; Amoros, Claude; Lamouroux, Nicolas (March 1998). "Aquatic plant diversity in riverine wetlands: the role of connectivity". Freshwater Biology. 39 (2): 267–283. Bibcode:1998FrBio..39..267B. doi:10.1046/j.1365-2427.1998.00273.x. ISSN 0046-5070.
- ^ Wick, Emma; Sandler, Hilary; Ghantous, Katherine (April 2021). "AQUATIC WEEDS: BIOLOGY, DIVERSITY, AND GROWER ASSESSMENT OF PRESENCE ON CRANBERRY FARMS" (PDF). UMass Extension.
- ^ "Dictionary.com | Meanings & Definitions of English Words". Dictionary.com. Retrieved 2024-12-11.
- ^ "Phytoplankton | Definition, Examples, & Facts | Britannica". www.britannica.com. 2024-11-29. Retrieved 2024-12-11.
- ^ "Dictionary.com | Meanings & Definitions of English Words". Dictionary.com. Retrieved 2024-12-11.
- ^ Westlake, D.F.; Kvĕt, J.; Szczepański, A (1998). The Production Ecology of Wetlands. Cambridge: Cambridge University Press.
- ^ a b Mommer, Liesje; Wolters-Arts, Mieke; Andersen, Charlotte; Visser, Eric J. W.; Pedersen, Ole (2007). "Submergence-induced leaf acclimation in terrestrial species varying in flooding tolerance". New Phytologist. 176 (2): 337–345. Bibcode:2007NewPh.176..337M. doi:10.1111/j.1469-8137.2007.02166.x. hdl:2066/36521. ISSN 1469-8137. PMID 17888115.
- ^ a b Okuda, Kazuo (2002-08-01). "Structure and phylogeny of cell coverings". Journal of Plant Research. 115 (4): 283–288. Bibcode:2002JPlR..115..283O. doi:10.1007/s10265-002-0034-x. ISSN 1618-0860. PMID 12582732. S2CID 33043901.
- ^ Sarkar, Purbasha; Bosneaga, Elena; Auer, Manfred (2009-09-01). "Plant cell walls throughout evolution: towards a molecular understanding of their design principles". Journal of Experimental Botany. 60 (13): 3615–3635. doi:10.1093/jxb/erp245. ISSN 0022-0957. PMID 19687127. Archived from the original on 2020-06-06. Retrieved 2020-06-06.
- ^ Vries, Jan de; Archibald, John M. (2018). "Plant evolution: landmarks on the path to terrestrial life". New Phytologist. 217 (4): 1428–1434. Bibcode:2018NewPh.217.1428D. doi:10.1111/nph.14975. ISSN 1469-8137. PMID 29318635.
- ^ Najrana, Tanbir; Sanchez-Esteban, Juan (2016-12-26). "Mechanotransduction as an Adaptation to Gravity". Frontiers in Pediatrics. 4: 140. doi:10.3389/fped.2016.00140. ISSN 2296-2360. PMC 5183626. PMID 28083527.
- ^ a b Sand-Jensen, Kaj (1989-07-01). "Environmental variables and their effect on photosynthesis of aquatic plant communities". Aquatic Botany. Photosynthesis and Photorespiration in Aquatic Organisms. 34 (1): 5–25. Bibcode:1989AqBot..34....5S. doi:10.1016/0304-3770(89)90048-X. ISSN 0304-3770.
- ^ Chambers, Patricia A. (September 1987). "Light and Nutrients in the Control of Aquatic Plant Community Structure. II. In Situ Observations". The Journal of Ecology. 75 (3): 621–628. Bibcode:1987JEcol..75..621C. doi:10.2307/2260194. JSTOR 2260194.
- ^ a b "Do macrophytes play a role in constructed treatment wetlands?". Water Science and Technology. 35 (5). 1997. doi:10.1016/s0273-1223(97)00047-4. ISSN 0273-1223.
- ^ Cowardin, Lewis M.; Carter, Virginia; Golet, Francis C.; Laroe, Edward T. (2005-07-15), "Classification of Wetlands and Deepwater Habitats of the United States", in Lehr, Jay H.; Keeley, Jack (eds.), Water Encyclopedia, John Wiley & Sons, Inc., pp. sw2162, doi:10.1002/047147844x.sw2162, ISBN 9780471478447, archived from the original on 2019-12-21, retrieved 2019-11-16
- ^ Maréchal, Eric (2019). "Marine and Freshwater Plants: Challenges and Expectations". Frontiers in Plant Science. 10: 1545. doi:10.3389/fpls.2019.01545. ISSN 1664-462X. PMC 6883403. PMID 31824548.
- ^ Nichols, Stanley A. (February 4, 1974). "Mechanical and Habitat Manipulation for Aquatic Plant Management: A Review of Techniques". Department of Natural Resources – via Google Books.
- ^ Quilliam, Richard S.; van Niekerk, Melanie A.; Chadwick, David R.; Cross, Paul; Hanley, Nick; Jones, Davey L.; Vinten, Andy J.A.; Willby, Nigel; Oliver, David M. (April 2015). "Can macrophyte harvesting from eutrophic water close the loop on nutrient loss from agricultural land?". Journal of Environmental Management. 152: 210–217. Bibcode:2015JEnvM.152..210Q. doi:10.1016/j.jenvman.2015.01.046. hdl:10023/6517. PMID 25669857.
- ^ Verhofstad, M.J.J.M.; Poelen, M.D.M.; van Kempen, M.M.L.; Bakker, E.S.; Smolders, A.J.P. (September 2017). "Finding the harvesting frequency to maximize nutrient removal in a constructed wetland dominated by submerged aquatic plants". Ecological Engineering. 106: 423–430. Bibcode:2017EcEng.106..423V. doi:10.1016/j.ecoleng.2017.06.012.
- ^ Vymazal, Jan (2013). "Emergent plants used in free water surface constructed wetlands: A review". Ecological Engineering. 61: 582–592. Bibcode:2013EcEng..61..582V. doi:10.1016/j.ecoleng.2013.06.023.
- ^ Hallin, Sara; Hellman, Maria; Choudhury, Maidul I.; Ecke, Frauke (2015). "Relative importance of plant uptake and plant associated denitrification for removal of nitrogen from mine drainage in sub-arctic wetlands". Water Research. 85: 377–383. Bibcode:2015WatRe..85..377H. doi:10.1016/j.watres.2015.08.060. PMID 26360231.
- ^ Zhu, Mengyuan; Zhu, Guangwei; Nurminen, Leena; Wu, Tingfeng; Deng, Jianming; Zhang, Yunlin; Qin, Boqiang; Ventelä, Anne-Mari (2015). "The Influence of Macrophytes on Sediment Resuspension and the Effect of Associated Nutrients in a Shallow and Large Lake (Lake Taihu, China)". PLOS ONE. 10 (6): e0127915. Bibcode:2015PLoSO..1027915Z. doi:10.1371/journal.pone.0127915. PMC 4452177. PMID 26030094.
- ^ Horppila, Jukka; Kaitaranta, Joni; Joensuu, Laura; Nurminen, Leena (2013). "Influence of emergent macrophyte (Phragmites australis) density on water turbulence and erosion of organic-rich sediment". Journal of Hydrodynamics. 25 (2): 288–293. Bibcode:2013JHyDy..25..288H. doi:10.1016/S1001-6058(13)60365-0. S2CID 120990795.
- ^ Thomaz, Sidinei M.; Dibble, Eric D.; Evangelista, Luiz R.; Higuti, Janet; Bini, Luis M. (2007). "Influence of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lagoons". Freshwater Biology. 53 (2): 358–367. doi:10.1111/j.1365-2427.2007.01898.x.
- ^ a b Brix, Hans (1994-02-01). "Functions of Macrophytes in Constructed Wetlands". Water Science and Technology. 29 (4): 71–78. Bibcode:1994WSTec..29...71B. doi:10.2166/wst.1994.0160. ISSN 0273-1223.
- ^ Chai, Tsun-Thai; Ooh, Keng-Fei; Quah, Yixian; Wong, Fai-Chu (2015). "Edible freshwater macrophytes: A source of anticancer and antioxidative natural products—a mini-review". Phytochemistry Reviews. 14 (3): 443–457. Bibcode:2015PChRv..14..443C. doi:10.1007/s11101-015-9399-z. S2CID 15597431.
- ^ Ooh, K. F.; Ong, H. C.; Wong, F. C.; Sit, N. W.; Chai, T. T. (2014). "High performance liquid chromatography profiling of health-promoting phytochemicals and evaluation of antioxidant, anti-lipoxygenase, iron chelating and anti-glucosidase activities of wetland macrophytes". Pharmacognosy Magazine. 10 (Suppl 3): S443–S455. doi:10.4103/0973-1296.139767. PMC 4189257. PMID 25298659.
- ^ a b Wiley (2005-09-09). Encyclopedia of Life Sciences (1 ed.). Wiley. doi:10.1002/9780470015902.a0020475. ISBN 978-0-470-01617-6.
- ^ Šraj-Kržič, Nina; Pongrac, Paula; Klemenc, Maja; Kladnik, Aleš; Regvar, Marjana; Gaberščik, Alenka (2006-11-01). "Mycorrhizal colonisation in plants from intermittent aquatic habitats". Aquatic Botany. 85 (4): 331–336. Bibcode:2006AqBot..85..331S. doi:10.1016/j.aquabot.2006.07.001. ISSN 0304-3770.
- ^ Gross, Elisabeth M. (May 2003). "Allelopathy of Aquatic Autotrophs". Critical Reviews in Plant Sciences. 22 (3–4): 313–339. Bibcode:2003CRvPS..22..313G. doi:10.1080/713610859. ISSN 0735-2689.
- ^ a b c HUSSNER, A (2012-06-07). "Alien aquatic plant species in European countries". Weed Research. 52 (4): 297–306. Bibcode:2012WeedR..52..297H. doi:10.1111/j.1365-3180.2012.00926.x. ISSN 0043-1737.
- ^ a b c d "Invasive non-native aquatic plants". Northern Ireland Direct Government Services. 9 November 2015. Retrieved 6 February 2022.
- ^ "Invasive Pennywort plant 'strangling' River Thames". BBC. 27 March 2018.
- ^ "Parrot's -feather". Plant Life. Archived from the original on 15 February 2021. Retrieved 6 February 2022.
- ^ Brunel, Sarah; Petter, Françoise; Fernandez-Galiano, Eladio; Smith, Ian (2009), Inderjit (ed.), "Approach of the European and Mediterranean Plant Protection Organization to the Evaluation and Management of Risks Presented by Invasive Alien Plants", Management of Invasive Weeds, Invading Nature – Springer Series In Invasion Ecology, vol. 5, Dordrecht: Springer Netherlands, pp. 319–343, doi:10.1007/978-1-4020-9202-2_16, ISBN 978-1-4020-9202-2, retrieved 2022-03-06









