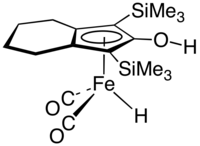
Knölker complex
Appearance
 | |
| Names | |
|---|---|
| IUPAC name
Dicarbonylhydro[(1,2,3,3a,7a-η)-4,5,6,7-tetrahydro-2-hydroxy-1,3-bis(trimethylsilyl)-1H-inden-1-yl]iron
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| C17H28FeO3Si2 | |
| Molar mass | 392.423 g·mol−1 |
| polar organic solvents | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The Knölker complex is an organoiron compound, which is a catalyst for transfer hydrogenation.[1] The complex features an hydroxycyclopentadienyl ligand bound to an Fe(CO)2H centre. It is generated by the corresponding cyclopentadienone tricarbonyl by treatment with base followed by acidification.[2] It is named for Hans-Joachim Knölker The compound is related to the organoruthenium compound called Shvo's complex, a hydroxycyclopentadienyl derivative that also functions as a catalyst for hydrogenation.[3]
References
[edit]- ^ Casey, Charles P.; Guan, Hairong (2007). "An Efficient and Chemoselective Iron Catalyst for the Hydrogenation of Ketones". Journal of the American Chemical Society. 129 (18): 5816–5817. doi:10.1021/ja071159f. PMID 17439131.
- ^ Knölker, Hans-Joachim; Baum, Elke; Goesmann, Helmut; Klauss, Rüdiger (1999). "Demetalation of Tricarbonyl(cyclopentadienone)iron Complexes Initiated by a Ligand Exchange Reaction with NaOH—X-Ray Analysis of a Complex with Nearly Square-Planar Coordinated Sodium". Angewandte Chemie International Edition. 38 (13–14): 2064–2066. doi:10.1002/(SICI)1521-3773(19990712)38:13/14<2064::AID-ANIE2064>3.0.CO;2-W. PMID 34182704.
- ^ Bullock, R. Morris (2007). "An Iron Catalyst for Ketone Hydrogenations under Mild Conditions". Angewandte Chemie International Edition. 46 (39): 7360–7363. doi:10.1002/anie.200703053. PMID 17847139.
