Kallikrein
Kallikreins are a subgroup of serine proteases, enzymes capable of cleaving peptide bonds in proteins. In humans, plasma kallikrein (encoded by KLKB1 gene) has no known paralogue, while tissue kallikrein-related peptidases (KLKs) encode a family of fifteen closely related serine proteases. These genes are localised to chromosome 19q13, forming the largest contiguous cluster of proteases within the human genome. Kallikreins are responsible for the coordination of various physiological functions including blood pressure, semen liquefaction and skin desquamation.
Occurrence
[edit]In 1934, Eugen Werle reported finding a substance in the pancreas of humans and various animals in such large amounts that the pancreas could be taken for its site of origin. He named it kallikrein, by derivation from the Greek word καλλίκρεας (kallíkreas) 'pancreas'. Since then, similar enzymes have been found in the biological fluids of humans and other mammals, as well as in some snake venoms.[1]
Venom
[edit]The caterpillar known as Lagoa crispata contains poison glands attached to hypodermic spines, which produce and inject venom that has been characterized as kallikrein in nature.[2]
The venom of solenodons and some shrews like the northern short-tailed shrew consist of multiple copies of kallikrein 1 (KLK1) serine proteases.[3] KLK1 are very similar to serine protease found in venomous snakes like vipers, and have evolved in parallel from a common toxin precursor,[4] which cause hypotensive effects in vivo.[5]
Plasma kallikrein
[edit]The KLKB1 gene encoding plasma kallikrein is located on chromosome 4q34-35. It is synthesised as an inactive precursor, prekallikrein, which must undergo proteolytic processing to become activated. This is facilitated by factor XII, PRCP or other stimuli.[citation needed]
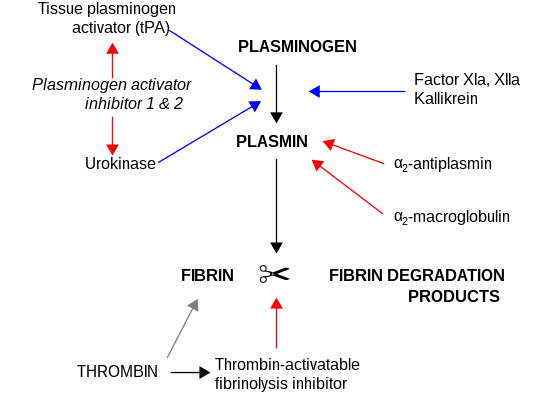
Plasma kallikrein liberates kinins (bradykinin and kallidin) from the kininogens,[6][7] peptides responsible for the regulation of blood pressure and activation of inflammation. It is also capable of generating plasmin from plasminogen:
Structure
[edit]Kallikrein is homologous to factor XI and consists of four apple domains and one serine protease domain.[citation needed]
Tissue kallikreins
[edit]Distinct from plasma kallikrein, tissue kallikreins (KLKs) are expressed throughout the human body and perform various physiological roles. As some kallikreins are able to catalyse the activation of other kallikreins, several cascades involving these proteases have been implicated in the regulation of homeostatic functions.[citation needed]
Function
[edit]Similar to KLKB1, three tissue kallikreins KLK1, KLK2 and KLK12 also participate in regulation of blood pressure via the activation of bradykinin.[8] KLK2, KLK3, KLK4, KLK5 and KLK14 are expressed in the prostate and are thought to be responsible for regulating semen liquefaction through hydrolysis of semenogelin.[9][10] Desquamation of the skin is likely controlled by KLK5, KLK7 and KLK14, which are expressed in the outermost layer of the epidermis and cleave cellular adhesion proteins.[11] Additionally, KLK6 and KLK8 are associated with neuronal plasticity in the central nervous system.[12]
Genes
[edit]There are 15 known human tissue kallikreins: KLK1, KLK2, KLK3, KLK4, KLK5, KLK6, KLK7, KLK8, KLK9, KLK10, KLK11, KLK12, KLK13, KLK14, KLK15.[citation needed]
Clinical significance
[edit]Kallikrein-related peptidases are targets of active investigation by drug researchers as possible biomarkers for cancer.[13][14]
Prostate-specific antigen (PSA; hk3, human kallikrein gene 3) and human glandular kallikrein (hK2) are used as tumor markers for prostate cancer.[citation needed]
Ecallantide, lanadelumab, and berotralstat are FDA-approved drugs that inhibit kallikrein and can be used for managing hereditary angioedema.
A missense variant on KLK15 has been associated with hypermobile Ehlers-Danlos syndrome.[15]
See also
[edit]References
[edit]- ^ Raspi G (September 1996). "Kallikrein and kallikrein-like proteinases: purification and determination by chromatographic and electrophoretic methods". Journal of Chromatography. B, Biomedical Applications. 684 (1–2): 265–287. doi:10.1016/0378-4347(96)00144-2. PMID 8906477.
- ^ Lamdin JM, Howell DE, Kocan KM, Murphey DR, Arnold DC, Fenton AW, et al. (September 2000). "The venomous hair structure, venom and life cycle of Lagoa crispata, a puss caterpillar of Oklahoma". Toxicon. 38 (9): 1163–1189. doi:10.1016/s0041-0101(99)00195-6. PMID 10736472.
- ^ Casewell NR, Petras D, Card DC, Suranse V, Mychajliw AM, Richards D, et al. (December 2019). "Solenodon genome reveals convergent evolution of venom in eulipotyphlan mammals". Proceedings of the National Academy of Sciences of the United States of America. 116 (51): 25745–25755. Bibcode:2019PNAS..11625745C. doi:10.1073/pnas.1906117116. PMC 6926037. PMID 31772017.
- ^ Barua A, Koludarov I, Mikheyev AS (December 2021). "Co-option of the same ancestral gene family gave rise to mammalian and reptilian toxins". BMC Biology. 19 (1): 268. doi:10.1186/s12915-021-01191-1. PMC 8705180. PMID 34949191.
- ^ Kita M, Okumura Y, Ohdachi SD, Oba Y, Yoshikuni M, Nakamura Y, et al. (February 2005). "Purification and characterisation of blarinasin, a new tissue kallikrein-like protease from the short-tailed shrew Blarina brevicauda: comparative studies with blarina toxin". Biological Chemistry. 386 (2): 177–182. doi:10.1515/BC.2005.022. hdl:2115/7398. PMID 15843162. S2CID 2884850.
- ^ Bhoola KD, Figueroa CD, Worthy K (March 1992). "Bioregulation of kinins: kallikreins, kininogens, and kininases". Pharmacological Reviews. 44 (1): 1–80. PMID 1313585.
- ^ Stefan Offermanns; Walter Rosenthal (2008). Encyclopedia of Molecular Pharmacology. Springer. pp. 673–. ISBN 978-3-540-38916-3. Retrieved 11 December 2010.
- ^ Giusti B, Serratì S, Margheri F, Papucci L, Rossi L, Poggi F, et al. (November 2005). "The antiangiogenic tissue kallikrein pattern of endothelial cells in systemic sclerosis". Arthritis and Rheumatism. 52 (11): 3618–3628. doi:10.1002/art.21383. PMID 16255054.
- ^ Michael IP, Pampalakis G, Mikolajczyk SD, Malm J, Sotiropoulou G, Diamandis EP (May 2006). "Human tissue kallikrein 5 is a member of a proteolytic cascade pathway involved in seminal clot liquefaction and potentially in prostate cancer progression". The Journal of Biological Chemistry. 281 (18): 12743–12750. doi:10.1074/jbc.M600326200. PMID 16517595.
- ^ Emami N, Diamandis EP (February 2008). "Human kallikrein-related peptidase 14 (KLK14) is a new activator component of the KLK proteolytic cascade. Possible function in seminal plasma and skin". The Journal of Biological Chemistry. 283 (6): 3031–3041. doi:10.1074/jbc.M707253200. PMID 18056261.
- ^ Ovaere P, Lippens S, Vandenabeele P, Declercq W (September 2009). "The emerging roles of serine protease cascades in the epidermis". Trends in Biochemical Sciences. 34 (9): 453–463. doi:10.1016/j.tibs.2009.08.001. PMID 19726197.
- ^ Tamura H, Ishikawa Y, Hino N, Maeda M, Yoshida S, Kaku S, Shiosaka S (February 2006). "Neuropsin is essential for early processes of memory acquisition and Schaffer collateral long-term potentiation in adult mouse hippocampus in vivo". The Journal of Physiology. 570 (Pt 3): 541–551. doi:10.1113/jphysiol.2005.098715. PMC 1479887. PMID 16308352.
- ^ Borgoño CA, Diamandis EP (November 2004). "The emerging roles of human tissue kallikreins in cancer". Nature Reviews. Cancer. 4 (11): 876–890. doi:10.1038/nrc1474. PMID 15516960. S2CID 382797.
- ^ Diamandis EP, Yousef GM (August 2002). "Human tissue kallikreins: a family of new cancer biomarkers". Clinical Chemistry. 48 (8): 1198–1205. doi:10.1093/clinchem/48.8.1198. PMID 12142373.
- ^ "Variants in the Kallikrein Gene Family and Hypermobile Ehlers-Danlos Syndrome".
External links
[edit]- The MEROPS online database for peptidases and their inhibitors: S01.212 Archived 2020-05-27 at the Wayback Machine
- Kallikreins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
