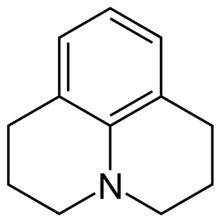
Julolidine
Appearance
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,3,6,7-Tetrahydro-1H,5H-pyrido[3,2,1-ij]quinoline | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.006.851 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H15N | |
| Molar mass | 173.259 g·mol−1 |
| Density | 1.003 g/mL |
| Melting point | 35 °C (95 °F; 308 K) |
Refractive index (nD)
|
1.568 |
| Hazards | |
| Flash point | 110 °C (230 °F; 383 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Julolidine is a heterocyclic aromatic organic compound. It has the formula C12H15N.
Synthesis
[edit]The first synthesis of julolidine was first reported by G. Pinkus in 1892.[1]
Applications
[edit]This compound and its derivatives have found recent interest as photoconductive materials, chemiluminescence substances, chromogenic substrates in analytical redox reactions, dye intermediates, potential antidepressants and tranquilizers, nonlinear optical materials, high sensitivity photopolymerizable materials, and for improving color stability in photography.
References
[edit]- ^ Pinkus, G. Ueber die Einwirkung von Trimethylenchlorbromid auf einige aromatische Amine und Amide. Berichte der deutschen chemischen Gesellschaft 1892, 25 (2), 2798–2806
External links
[edit]- Katritzky, Alan R.; Rachwal, Bogumila; Rachwal, Stanislaw; Abboud, Khalil A. (1996). "Convenient Synthesis of Julolidines Using Benzotriazole Methodology". The Journal of Organic Chemistry. 61 (9): 3117. doi:10.1021/jo9519118. PMID 11667174.
