Hexafluoroacetone

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,1,3,3,3-Hexafluoropropan-2-one | |
| Other names
perfluoroacetone
acetone hexafluoride perfluoro-2-propanone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.616 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2420 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3F6O | |
| Molar mass | 166.02 g/mol |
| Appearance | Colorless gas |
| Odor | musty[1] |
| Density | 1.32 g/ml, liquid |
| Melting point | −129 °C (144 K) |
| Boiling point | −28 °C (245 K) |
| Reacts with water | |
| Vapor pressure | 5.8 atm (20 °C)[1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic (T), Corrosive (C) |
| GHS labelling: | |
   
| |
| Danger | |
| H301, H310, H311, H314, H315, H330, H360, H370, H372 | |
| P201, P202, P260, P262, P264, P270, P271, P280, P281, P284, P301+P310, P301+P330+P331, P302+P350, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P307+P311, P308+P313, P310, P312, P314, P320, P321, P322, P330, P332+P313, P361, P362, P363, P403+P233, P405, P410+P403, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Nonflammable[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[1] |
REL (Recommended)
|
TWA 0.1 ppm (0.7 mg/m3) [skin][1] |
IDLH (Immediate danger)
|
N.D.[1] |
| Related compounds | |
Related ketones;
organofluorides |
Acetone; Hexafluoro-2-propanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
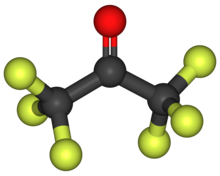
Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It is a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a musty odour.[2] According to electron diffraction, HFA and acetone adopt very similar structures, the C-O distance being only longer in the fluorinated compound (124.6 vs 121.0 pm), possibly due to steric effects.[3]
The term "hexafluoroacetone" can refer to the sesquihydrate (1.5 H2O), which is a hemihydrate of hexafluoropropane-2,2-diol (F
3C)
2C(OH)
2, a geminal diol. Hydrated HFA behaves differently from the anhydrous material.
Synthesis
[edit]The industrial route to HFA involves treatment of hexachloroacetone with HF (a Finkelstein reaction):[4]
- (CCl3)2CO + 6 HF → (CF3)2CO + 6 HCl
Laboratory methods
[edit]Hydrated HFA can be converted to HFA by treatment with hot sulfuric acid.[5]
It has also be prepared from hexafluoropropylene oxide, which will rearrange to give HFA when heated in the presence of a Lewis acid such as AlCl3.[6] The Lewis acid catalysed oxidation of hexafluoropropylene will also produce HFA, via a similar mechanism.
Although it is commercially available, HFA can be prepared on the laboratory-scale from hexafluoropropylene.[7] In the first step KF catalyzes the reaction of the alkene with elemental sulfur to give the 1,3-dithietane dimer of hexafluorothioacetone. This species is then oxidized by potassium iodate to give HFA.[8]
Uses
[edit]Hexafluoroacetone is used in the production of hexafluoroisopropanol:
- (CF3)2CO + H2 → (CF3)2CHOH
It is also used as a precursor to hexafluoroisobutylene,[4] a monomer used in polymer chemistry, and as a building block in the synthesis of midaflur, bisphenol AF, 4,4′-(hexafluoroisopropylidene)diphthalic anhydride, and alitame.
Reactivity
[edit]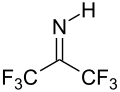
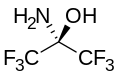
With water, hexafluoroacetone converts to the hydrate. The equilibrium constant (Keq) for the formation of this geminal diol is 106 M−1. The analogous equilibrium for acetone is an unfavorable 10−3 M−1.[9] Hexafluoroacetone-hydrates are acidic. In an analogous reaction, ammonia adds to hexafluoroacetone to give the hemiaminal (CF3)2C(OH)(NH2) which can be dehydrated with phosphoryl chloride to give the imine (CF3)2CNH.[10]
Nucleophiles attack occurs at the carbonyl carbon of Hexafluoroacetone, as illustrated above. Thus, HFA readily forms lactones when treated with hydroxy- and amine-substituted carboxylic acids. In such reactions, HFA serves both as electrophile and dehydrating agent:[5]
- RCH(OH)CO2H + O=C(CF3)2 → RCH(O)CO2C(CF3)2 + (HO)2C(CF3)2
See also
[edit]References
[edit]- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0319". National Institute for Occupational Safety and Health (NIOSH).
- ^ CDC - NIOSH Pocket Guide to Chemical Hazards
- ^ Hilderbrandt, R. L.; Andreassen, A. L.; Bauer, Simon Harvey (1970). "Electron diffraction investigation of hexafluoroacetone, hexafluoropropylimine, and hexafluoroisobutene". The Journal of Physical Chemistry. 74 (7): 1586–1592. doi:10.1021/j100702a030.
- ^ a b Günter Siegemund; Werner Schwertfeger; Andrew Feiring; Bruce Smart; Fred Behr; Herward Vogel; Blaine McKusick (2002). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349.
- ^ a b Spengler, Jan; Böttcher, Christoph; Albericio, Fernando; Burger, Klaus (2006). "Hexafluoroacetone as Protecting and Activating Reagent: New Routes to Amino, Hydroxy, and Mercapto Acids and Their Application for Peptide and Glyco- and Depsipeptide Modification". Chemical Reviews. 106 (11): 4728–4746. doi:10.1021/cr0509962. PMID 17091933.
- ^ Millauer, Hans; Schwertfeger, Werner; Siegemund, Günter (March 1985). "Hexafluoropropene Oxide — A Key Compound in Organofluorine Chemistry". Angewandte Chemie International Edition in English. 24 (3): 161–179. doi:10.1002/anie.198501611.
- ^ Anello, Louis G.; Van der Puy, Michael (January 1982). "A convenient synthesis of hexafluoroacetone". The Journal of Organic Chemistry. 47 (2): 377–378. doi:10.1021/jo00341a046.
- ^ Van Der Puy, M.; Anello, L. G. (1985). "Hexafluoroacetone". Organic Syntheses. 53: 154. doi:10.15227/orgsyn.063.015.
- ^ Lemal, David M. (2004). "Perspective on Fluorocarbon Chemistry". The Journal of Organic Chemistry. 69 (1): 1–11. doi:10.1021/jo0302556. PMID 14703372.
- ^ W. J. Middleton; H. D. Carlson (1970). "Hexafluoroacetone imine". Org. Syntheses. 50: 81–3. doi:10.15227/orgsyn.050.0081..

