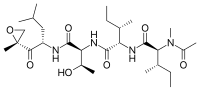
Epoxomicin
Appearance
 | |
| Names | |
|---|---|
| IUPAC name
(2S,3S)-N-((2S,3R)-3-hydroxy-1-(((S)-4-methyl-1-((R)-2-methyloxiran-2-yl)-1-oxopentan-2-yl)amino)-1-oxobutan-2-yl)-3-methyl-2-((2S,3S)-3-methyl-2-(N-methylacetamido)pentanamido)pentanamide
| |
| Other names
BU 4061T
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H50N4O7 | |
| Molar mass | 554.729 g·mol−1 |
| Appearance | White solid |
| Solubility in DMSO | 10 mg/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Epoxomicin is a naturally occurring selective proteasome inhibitor with anti-inflammatory activity.[2] It was originally discovered in 1992.[3] Injected, it can induce Parkinson's-like symptoms in rats.
Derivatives of epoxomicin include carfilzomib.
References
[edit]- ^ Epoxomicin, Santa Cruz Biotechnology
- ^ Meng, L; Mohan, R; Kwok, BH; Elofsson, M; Sin, N; Crews, CM (1999). "Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity". PNAS. 96 (18): 10403–10408. Bibcode:1999PNAS...9610403M. doi:10.1073/pnas.96.18.10403. PMC 17900. PMID 10468620.
- ^ Epoxomicin, Peptide Institute, Inc.
Further reading
[edit]- Hanada, M; Sugawara, K; Kaneta, K; Toda, S; Nishiyama, Y; Tomita, K; Yamamoto, H; Konishi, M; Oki, T (November 1992). "Epoxomicin, a new antitumor agent of microbial origin". J. Antibiot. 45 (11): 1746–52. doi:10.7164/antibiotics.45.1746. PMID 1468981.
- Meng, L.; et al. (1999). "Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity". Proc. Natl. Acad. Sci. U.S.A. 96 (18): 10403–10408. Bibcode:1999PNAS...9610403M. doi:10.1073/pnas.96.18.10403. PMC 17900. PMID 10468620.
- Schwarz, K.; et al. (2000). "The selective proteasome inhibitors lactacystin and epoxomicin can be used to either up- or down-regulate antigen presentation at nontoxic doses". J. Immunol. 164 (12): 6147–6157. doi:10.4049/jimmunol.164.12.6147. PMC 2507740. PMID 10843664.
- Princiotta, M.F.; et al. (2001). "Cells adapted to the proteasome inhibitor 4-hydroxy- 5-iodo-3-nitrophenylacetyl-Leu-Leu-leucinal-vinyl sulfone require enzymatically active proteasomes for continued survival". Proc. Natl. Acad. Sci. U.S.A. 98 (2): 513–518. doi:10.1073/pnas.021132398. PMC 14618. PMID 11149939.
- Garrett, I.R.; et al. (2003). "Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro". J. Clin. Invest. 111 (11): 1771–1782. doi:10.1172/JCI16198. PMC 156102. PMID 12782679.
- McNaught, K.S.; et al. (2004). "Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson's disease". Annals of Neurology. 56 (1): 149–162. doi:10.1002/ana.20186. PMID 15236415. S2CID 25314945.
