Draft:Jamal Lasri
 | Review waiting, please be patient.
This may take 8 weeks or more, since drafts are reviewed in no specific order. There are 1,799 pending submissions waiting for review.
Where to get help
How to improve a draft
You can also browse Wikipedia:Featured articles and Wikipedia:Good articles to find examples of Wikipedia's best writing on topics similar to your proposed article. Improving your odds of a speedy review To improve your odds of a faster review, tag your draft with relevant WikiProject tags using the button below. This will let reviewers know a new draft has been submitted in their area of interest. For instance, if you wrote about a female astronomer, you would want to add the Biography, Astronomy, and Women scientists tags. Editor resources
Reviewer tools
|
Jamal Lasri | |
|---|---|
 | |
| Born | |
| Nationality | |
| Citizenship | |
| Alma mater | University of Valencia University of Hassan II Casablanca |
| Scientific career | |
| Fields | Organic Chemistry, Coordination Chemistry and Catalysis |
| Institutions | King Abdulaziz University, Jeddah Instituto Superior Técnico, University of Lisbon |
| Doctoral advisor | Prof. José Sepúlveda-Arques http://www.uv.es/sepulved/ |
Jamal Lasri (in Arabic: جمال العسري) was born in 1973 in Casablanca, is a Portuguese-Moroccan synthetic Chemist. He received his B.Sc. degree in Chemistry from the University of Hassan II Casablanca (Morocco) in 1997. Then, he moved to Spain for his M.Sc. and Ph.D. studies at the Faculty of Pharmacy of the University of Valencia under the supervision of Prof. José Sepúlveda-Arques.[1] His work was focused on the synthesis of pyrido[3,2-d]pyrimidine-2,4-diones,[2] novel route for the synthesis of N-tosylguanidines by ring cleavage of 1,2-dihydro-2-tosyliminopyrimidines[3],[4] and study of the intermolecular transamidation reactions of N-carbamoylmethyl-N’-tosylguanidines with primary amines.[5],[6] He obtained his M.Sc. and Ph.D. degrees in organic chemistry in 2002 and 2004, respectively (Excellent "Cum Laude").[7]
Career
[edit]In September 2004, he started his postdoctoral research funded by a fellowship from Fundación Bancaja - Caja Castellón at the Jaume I University of Castellón (Spain) joining the group of Prof. Santiago Vicente Luis Lafuente.[8] He worked on the design and synthesis of new copper bis(oxazoline) polymeric systems and their use as heterogeneous catalysts for cyclopropanation reactions.
In May 2005, he moved to Portugal to work as a postdoctoral researcher with a Fundação para a Ciência e Tecnologia fellowship in the group of Prof. Armando J. L. Pombeiro at the Instituto Superior Técnico of the University of Lisbon.
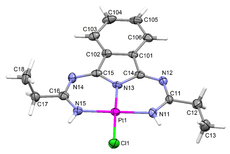
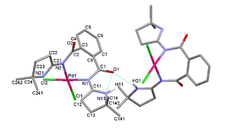
In January 2008, he was appointed associate researcher (Science 2007 program) at the Instituto Superior Técnico. His research interests involved the activation of organonitriles by transition metal centers, metal-mediated synthesis of N-heterocyclic compounds via [2+3] cycloaddition,[9],[10] thermal and microwave-assisted transition metal-catalyzed cross-coupling reactions for carbon-carbon bond construction (e.g. Suzuki-Miyaura, Mizoroki–Heck types) in water,[11],[12] peroxidative oxidation of alcohols,[13],[14] alkanes[15],[16] and green chemistry.
In September 2013, he holds a position of associate professor of organic chemistry at King Abdulaziz University (Saudi Arabia). Since June 2015, he is Full Professor of organic chemistry.
Lasri discovered that azines are also produced when chalcone reacts with a hydrazone to furnish 3,5-diphenyl-1H-pyrazole.[17]. In addition, together with Armando J. L. Pombeiro and João J. R. Fraústo da Silva they reported the first example of oxadiazoline and ketoimine palladium(II) complexes as highly efficient catalytic systems for Suzuki reaction in supercritical carbon dioxide (scCO2).[18]
References
[edit]- ^ http://www.uv.es/sepulved/
- ^ R. Mamouni, M. Aadil, M. Akssira, J. Lasri, J. Sepúlveda. Tetrahedron Lett. 2003, 44, 2745. http://dx.doi.org/10.1016/S0040-4039(03)00273-9
- ^ J. Lasri, M. E. González, J. Sepúlveda. Synthesis 2003, 845. http://dx.doi.org/10.1055/s-2003-38684
- ^ P. Fernandez, A. Ubeda, I. Guillén, J. Lasri, M. E. González, M. Akssira, J. Sepúlveda. Eur. J. Med. Chem. 2003, 38, 289. http://dx.doi.org/10.1016/S0223-5234(03)00013-8
- ^ M. E. González, E. Castillo, J. Lasri, J. Sepúlveda. Prog. React. Kinet. Mech. 2004, 29, 311.
- ^ J. Lasri, M. E. González, J. Sepúlveda. Org. Lett. 2003, 5, 3851. http://dx.doi.org/10.1021/ol035377z
- ^ https://dialnet.unirioja.es/servlet/tesis?codigo=217687
- ^ http://www.uji.es/CA/departaments/qio/estructura/personal/e@/22752?p_per_id=65302
- ^ J. Lasri, M. N. Kopylovich, M. F. C. Guedes da Silva, M. A. Januário Charmier, A. J. L. Pombeiro, Chem. -Eur. J. 2008, 14, 9312. http://dx.doi.org/10.1002/chem.200800510
- ^ J. Lasri, M. A. Januário Charmier, M. Haukka, A. J. L. Pombeiro, J. Org. Chem. 2007, 72, 750. http://dx.doi.org/10.1021/jo061659b
- ^ J. Lasri, M. F. C. Guedes da Silva, M. N. Kopylovich, S. Mukhopadhyay, M. A. Januário Charmier, A. J. L. Pombeiro, Dalton Trans. 2009, 2210. http://dx.doi.org/10.1039/b813996b
- ^ J. Lasri, T. C. O. Mac Leod, A. J. L. Pombeiro, Appl. Catal. A: Gen. 2011, 397, 94. http://dx.doi.org/10.1016/j.apcata.2011.02.019
- ^ P. J. Figiel, M. N. Kopylovich, J. Lasri, M. F. C. Guedes da Silva, J. J. R. Fraústo da Silva, A. J. L. Pombeiro, Chem Commun. 2010, 46, 2766. http://dx.doi.org/10.1039/b922738e
- ^ M. N. Kopylovich, Y. Yu. Karabach, M. F. C. Guedes da Silva, P. J. Figiel, J. Lasri, A. J. L. Pombeiro, Chem. -Eur. J. 2012, 18, 899. http://dx.doi.org/10.1002/chem.201101688
- ^ R. R. Fernandes, J. Lasri, M. F. C. Guedes da Silva, J. A. L. da Silva, J. J. R. Fraústo da Silva, A. J. L. Pombeiro, Appl. Catal. A: Gen. 2011, 402, 110. http://dx.doi.org/10.1016/j.apcata.2011.05.035
- ^ R. R. Fernandes, J. Lasri, A. M. Kirillov, M. F. C. Guedes da Silva, J. A. L. da Silva, J. J. R. Fraústo da Silva, A. J. L. Pombeiro, Eur. J. Inorg. Chem. 2011, 3781. http://dx.doi.org/10.1002/ejic.201100460
- ^ J. Lasri, A. I. Ismail, Indian J. Chem. Sect. B. 2018, 57B, 362. http://nopr.niscpr.res.in/handle/123456789/43824
- ^ R. R. Fernandes, J. Lasri, M. F. C. Guedes da Silva, A. M. F. Palavra, J. A. L. da Silva, J. J. R. Fraústo da Silva, A. J. L. Pombeiro, Adv. Synth. Catal. 2011, 353, 1153. http://dx.doi.org/10.1002/adsc.201000909
