Cystathionine gamma-synthase
| cystathionine gamma-synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Cystathionine gamma-synthase homotetramer, Helicobacter pylori | |||||||||
| Identifiers | |||||||||
| EC no. | 2.5.1.48 | ||||||||
| CAS no. | 9030-70-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
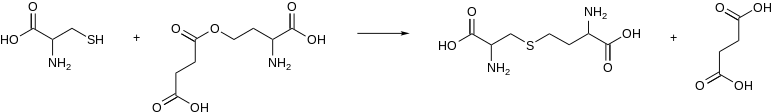
In enzymology, a cystathionine gamma-synthase (EC 2.5.1.48) is an enzyme that catalyzes the formation of cystathionine from cysteine and an activated derivative of homoserine, e.g.:
In microorganisms, the activated substrate of this enzyme is O4-succinyl-L-homoserine or O4-acetyl-L-homoserine. Cystathionine gamma-synthase from plants uses L-homoserine phosphate instead.[1]
This enzyme belongs to the family of transferases, specifically those transferring aryl or alkyl groups other than methyl groups. The systematic name of this enzyme class is O4-succinyl-L-homoserine:L-cysteine S-(3-amino-3-carboxypropyl)transferase. Other names in common use include O-succinyl-L-homoserine succinate-lyase (adding cysteine), O-succinylhomoserine (thiol)-lyase, homoserine O-transsuccinylase, O-succinylhomoserine synthase, O-succinylhomoserine synthetase, cystathionine synthase, cystathionine synthetase, homoserine transsuccinylase, 4-O-succinyl-L-homoserine:L-cysteine, and S-(3-amino-3-carboxypropyl)transferase. This enzyme participates in 4 metabolic pathways: methionine metabolism, cysteine metabolism, selenoamino acid metabolism, and sulfur metabolism. It employs one cofactor, pyridoxal phosphate.
References
[edit]- ^ Steegborn C, Laber B, Messerschmidt A, Huber R, Clausen T (August 2001). "Crystal structures of cystathionine gamma-synthase inhibitor complexes rationalize the increased affinity of a novel inhibitor". Journal of Molecular Biology. 311 (4): 789–801. doi:10.1006/jmbi.2001.4880. PMID 11518531.
- Flavin M, Slaughter C (March 1967). "Enzymatic synthesis of homocysteine or methionine directly from O-succinyl-homoserine". Biochimica et Biophysica Acta. 132 (2): 400–5. doi:10.1016/0005-2744(67)90158-1. PMID 5340123.
- Kaplan MM, Flavin M (October 1966). "Cystathionine gamma-synthetase of Salmonella. Catalytic properties of a new enzyme in bacterial methionine biosynthesis". The Journal of Biological Chemistry. 241 (19): 4463–71. doi:10.1016/S0021-9258(18)99743-7. PMID 5922970.
- Wiebers JL, Garner HR (January 1967). "Homocysteine and cysteine synthetases of Neurospora crassa. Purification, properties, and feedback control of activity". The Journal of Biological Chemistry. 242 (1): 12–23. doi:10.1016/S0021-9258(18)96312-X. PMID 6016326.
- Wiebers JL, Garner HR (December 1967). "Acyl derivatives of homoserine as substrates for homocysteine synthesis in Neurospora crassa, yeast, and Escherichia coli". The Journal of Biological Chemistry. 242 (23): 5644–9. doi:10.1016/S0021-9258(18)99405-6. PMID 12325384.
- Clausen T, Huber R, Prade L, Wahl MC, Messerschmidt A (December 1998). "Crystal structure of Escherichia coli cystathionine gamma-synthase at 1.5 A resolution". The EMBO Journal. 17 (23): 6827–38. doi:10.1093/emboj/17.23.6827. PMC 1171030. PMID 9843488.
- Ravanel S, Gakière B, Job D, Douce R (April 1998). "Cystathionine gamma-synthase from Arabidopsis thaliana: purification and biochemical characterization of the recombinant enzyme overexpressed in Escherichia coli". The Biochemical Journal. 331 ( Pt 2) (2): 639–48. doi:10.1042/bj3310639. PMC 1219399. PMID 9531508.