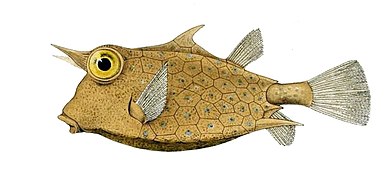
Fish scale

A fish scale is a small rigid plate that grows out of the skin of a fish. The skin of most jawed fishes is covered with these protective scales, which can also provide effective camouflage through the use of reflection and colouration, as well as possible hydrodynamic advantages. The term scale derives from the Old French escale, meaning a shell pod or husk.[1]
Scales vary enormously in size, shape, structure, and extent, ranging from strong and rigid armour plates in fishes such as shrimpfishes and boxfishes, to microscopic or absent in fishes such as eels and anglerfishes. The morphology of a scale can be used to identify the species of fish it came from. Scales originated within the jawless ostracoderms, ancestors to all jawed fishes today. Most bony fishes are covered with the cycloid scales of salmon and carp, or the ctenoid scales of perch, or the ganoid scales of sturgeons and gars. Cartilaginous fishes (sharks and rays) are covered with placoid scales. Some species are covered instead by scutes, and others have no outer covering on part or all of the skin.
Fish scales are part of the fish's integumentary system, and are produced from the mesoderm layer of the dermis, which distinguishes them from reptile scales.[2][3] The same genes involved in tooth and hair development in mammals are also involved in scale development. The placoid scales of cartilaginous fishes are also called dermal denticles and are structurally homologous with vertebrate teeth. Most fish are also covered in a layer of mucus or slime which can protect against pathogens such as bacteria, fungi, and viruses, and reduce surface resistance when the fish swims.
Thelodont scales
[edit]
The bony scales of thelodonts, the most abundant form of fossil fish, are well understood. The scales were formed and shed throughout the organisms' lifetimes, and quickly separated after their death.[4]
Bone, a tissue that is both resistant to mechanical damage and relatively prone to fossilization, often preserves internal detail, which allows the histology and growth of the scales to be studied in detail. The scales comprise a non-growing "crown" composed of dentine, with a sometimes-ornamented enameloid upper surface and an aspidine base.[5] Its growing base is made of cell-free bone, which sometimes developed anchorage structures to fix it in the side of the fish.[6] Beyond that, there appear to be five types of bone growth, which may represent five natural groupings within the thelodonts—or a spectrum ranging between the end members meta- (or ortho-) dentine and mesodentine tissues.[7] Each of the five scale morphs appears to resemble the scales of more derived groupings of fish, suggesting that thelodont groups may have been stem groups to succeeding clades of fish.[6]
However, using scale morphology alone to distinguish species has some pitfalls. Within each organism, scale shape varies hugely according to body area,[8] with intermediate forms appearing between different areas—and to make matters worse, scale morphology may not even be constant within one area. To confuse things further, scale morphologies are not unique to taxa, and may be indistinguishable on the same area of two different species.[9]
The morphology and histology of thelodonts provides the main tool for quantifying their diversity and distinguishing between species, although ultimately using such convergent traits is prone to errors. Nonetheless, a framework comprising three groups has been proposed based upon scale morphology and histology.[7] Comparisons to modern shark species have shown that thelodont scales were functionally similar to those of modern cartilaginous fish, and likewise has allowed an extensive comparison between ecological niches.[10]
Cosmoid scales
[edit]
Cosmoid scales are found only on ancient lobe-finned fishes, including some of the earliest lungfishes (subclass Dipnoi), and in Crossopterygii, including the living coelacanth in a modified form (see elasmoid scales, below). They were probably derived from a fusion of placoid-ganoid scales. The inner part of the scales is made of dense lamellar bone called isopedine. On top of this lies a layer of spongy or vascular bone supplied with blood vessels, followed by a complex dentine-like layer called cosmine with a superficial outer coating of vitrodentine. The upper surface is keratin. Cosmoid scales increase in size through the growth of the lamellar bone layer.[11]
Elasmoid scales
[edit]
Elasmoid scales are thin, imbricated scales composed of a layer of dense, lamellar collagen bone called isopedine, above which is a layer of tubercles usually composed of bone, as in Eusthenopteron. The layer of dentine that was present in the first lobe-finned fish is usually reduced, as in the extant coelacanth, or entirely absent, as in extant lungfish and in the Devonian Eusthenopteron.[12] Elasmoid scales have appeared several times over the course of fish evolution. They are present in some lobe-finned fishes, such as all extant and some extinct lungfishes, as well as the coelacanths which have modified cosmoid scales that lack cosmine and are thinner than true cosmoid scales. They are also present in some tetrapodomorphs like Eusthenopteron, amiids, and teleosts, whose cycloid and ctenoid scales represent the least mineralized elasmoid scales.
The zebrafish elasmoid scales are used in the lab to study bone mineralization process, and can be cultured (kept) outside of the organism.[13][14]
Ganoid scales
[edit]

Ganoid scales are found in the sturgeons, paddlefishes, gars, bowfin, and bichirs. They are derived from cosmoid scales and often have serrated edges. They are covered with a layer of hard enamel-like dentine in the place of cosmine, and a layer of inorganic bone salt called ganoine in place of vitrodentine.
Ganoine is a characteristic component of ganoid scales. It is a glassy, often multi-layered mineralized tissue that covers the scales, as well as the cranial bones and fin rays in some non-teleost ray-finned fishes,[15] such as gars, bichirs, and coelacanths.[16][17] It is composed of rod-like apatite crystallites.[18] Ganoine is an ancient feature of ray-finned fishes, being found for example on the scales of stem group actinopteryigian Cheirolepis.[17] While often considered a synapomorphic character of ray-finned fishes, ganoine or ganoine-like tissues are also found on the extinct acanthodii.[17] It has been suggested ganoine is homologous to tooth enamel in vertebrates[15] or even considered a type of enamel.[18]
 Amblypterus striatus
|
Ganoid scales of the extinct Carboniferous fish, Amblypterus striatus. (a) shows the outer surface of four of the scales, and (b) shows the inner surface of two of the scales. Each of the rhomboidal-shaped ganoid scales of Amblypterus has a ridge on the inner surface which is produced at one end into a projecting peg which fits into a notch in the next scale, similar to the manner in which tiles are pegged together on the roof of a house. | 
|
Most ganoid scales are rhomboidal (diamond-shaped) and connected by peg-and-socket joints. They are usually thick and fit together more like a jigsaw rather than overlapping like other scales.[19] In this way, ganoid scales are nearly impenetrable and are excellent protection against predation.
-
The sturgeon has rows of ganoid scales enlarged into scute-like armour plates.
-
The ganoid scales on a bowfin are reduced in size and resemble cycloid scales.

In sturgeons, the scales are greatly enlarged into armour plates along the sides and back, while in the bowfin the scales are greatly reduced in thickness to resemble cycloid scales.
-
Earrings made from the ganoid scales of an alligator gar
-
Fossil of a primitive rayfin with ganoid scales
Native Americans and people of the Caribbean used the tough ganoid scales of the alligator gar for arrow heads, breastplates, and as shielding to cover plows. In current times jewellery is made from these scales.[20]
Leptoid scales
[edit]Leptoid (bony-ridge) scales are found on higher-order bony fish, the teleosts (the more derived clade of ray-finned fishes). The outer part of these scales fan out with bony ridges while the inner part is criss-crossed with fibrous connective tissue. Leptoid scales are thinner and more translucent than other types of scales, and lack the hardened enamel-like or dentine layers. Unlike ganoid scales, further scales are added in concentric layers as the fish grows.[21]
Leptoid scales overlap in a head-to-tail configuration, like roof tiles, making them more flexible than cosmoid and ganoid scales. This arrangement allows a smoother flow of water over the body, and reduces drag.[22] The scales of some species exhibit bands of uneven seasonal growth called annuli (singular annulus). These bands can be used to age the fish.
Leptoid scales come in two forms: cycloid (smooth) and ctenoid (comb-like).[23]
Cycloid scales
[edit]Cycloid (circular) scales have a smooth texture and are uniform, with a smooth outer edge or margin. They are most common on fish with soft fin rays, such as salmon and carp.

|

|
Asian arowana have large cycloid scales arranged on the fish in a mosaic of raised ribs (left). The scales themselves are covered with a delicate net pattern (right).[24][25]
| |
Cycloid (circular) scales are usually found on carp-like or salmon-like fishes.
|
Ctenoid scales
[edit]Ctenoid (toothed) scales are like cycloid scales, except they have small teeth or spinules called ctenii along their outer or posterior edges. Because of these teeth, the scales have a rough texture. They are usually found on fishes with spiny fin rays, such as the perch-like fishes. These scales contain almost no bone, being composed of a surface layer containing hydroxyapatite and calcium carbonate and a deeper layer composed mostly of collagen. The enamel of the other scale types is reduced to superficial ridges and ctenii.

|

|
The size of the teeth on ctenoid scales can vary with position, as these scales from the rattail Cetonurus crassiceps show.
| |


Ctenoid (toothed) scales are usually found on perch-like fishes.
|
Ctenoid scales, similar to other epidermal structures, originate from placodes and distinctive cellular differentiation makes them exclusive from other structures that arise from the integument.[27] Development starts near the caudal fin, along the lateral line of the fish.[28] The development process begins with an accumulation of fibroblasts between the epidermis and dermis.[27] Collagen fibrils begin to organize themselves in the dermal layer, which leads to the initiation of mineralization.[27] The circumference of the scales grows first, followed by thickness when overlapping layers mineralize together.[27]
Ctenoid scales can be further subdivided into three types:
- Crenate scales, where the margin of the scale bears indentations and projections.
- Spinoid scales, where the scale bears spines that are continuous with the scale itself.
- True ctenoid scales, where the spines on the scale are distinct structures.
Most ray-finned fishes have ctenoid scales. Some species of flatfishes have ctenoid scales on the eyed side and cycloid scales on the blind side, while other species have ctenoid scales in males and cycloid scales in females.
Reflection
[edit]


Many teleost fish are covered with highly reflective scales which function as small mirrors and give the appearance of silvered glass. Reflection through silvering is widespread or dominant in fish of the open sea, especially those that live in the top 100 metres. A transparency effect can be achieved by silvering to make an animal's body highly reflective. At medium depths at sea, light comes from above, so a mirror oriented vertically makes animals such as fish invisible from the side.[29]
The marine hatchetfish is extremely flattened laterally (side to side), leaving the body just millimetres thick, and the body is so silvery as to resemble aluminium foil. The mirrors consist of microscopic structures similar to those used to provide structural coloration: stacks of between 5 and 10 crystals of guanine spaced about ¼ of a wavelength apart to interfere constructively and achieve nearly 100 per cent reflection. In the deep waters that the hatchetfish lives in, only blue light with a wavelength of 500 nanometres percolates down and needs to be reflected, so mirrors 125 nanometres apart provide good camouflage.[29]
Most fish in the upper ocean are camouflaged by silvering. In fish such as the herring, which lives in shallower water, the mirrors must reflect a mixture of wavelengths, and the fish accordingly has crystal stacks with a range of different spacings. A further complication for fish with bodies that are rounded in cross-section is that the mirrors would be ineffective if laid flat on the skin, as they would fail to reflect horizontally. The overall mirror effect is achieved with many small reflectors, all oriented vertically.[29]
Fish scales with these properties are used in some cosmetics, since they can give a shimmering effect to makeup and lipstick.[30]
Placoid scales
[edit]
Placoid (pointed, tooth-shaped) scales are found in the cartilaginous fishes: sharks, rays. They are also called dermal denticles. Placoid scales are structurally homologous with vertebrate teeth ("denticle" translates to "small tooth"), having a central pulp cavity supplied with blood vessels, surrounded by a conical layer of dentine, all of which sits on top of a rectangular basal plate that rests on the dermis. The outermost layer is composed of vitrodentine, a largely inorganic enamel-like substance. Placoid scales cannot grow in size, but rather more scales are added as the fish increases in size.
Similar scales can also be found under the head of the denticle herring. The amount of scale coverage is much less in rays.
Rhomboidal scales with the properties of both placoid and ganoid scales are suspected to exist in modern jawed fish ancestors: jawless ostracoderms and then jawed placoderms.
Shark skin
[edit]
Shark skin is almost entirely covered by small placoid scales. The scales are supported by spines, which feel rough when stroked in a backward direction, but when flattened by the forward movement of water, create tiny vortices that reduce hydrodynamic drag and reduce turbulence, making swimming both more efficient and quieter compared to that of bony fishes.[31] It also serves a role in anti-fouling by exhibiting the lotus effect.[32]
All denticles are composed of an interior pulp cavity with a nervous and arterial supply rooted in the dermis to supply the denticle with mucus.[33] Denticles contain riblet structures that protrude from the surface of the scale; under a microscope this riblet can look like a hook or ridges coming out of the scale. The overall shape of the protrusion from the denticle is dependent on the type of shark and can be generally described with two appearances.[34] The first is a scale in which ridges are placed laterally down the shark and parallel with the flow of the water. The second form is a smooth scale with what looks like a hooked riblet curling out of the surface aiming towards the posterior side of the shark.[34] Both riblet shapes assist in creating a turbulent boundary layer forcing the laminar flow farther away from the sharks skin.[35]
Unlike bony fish, sharks have a complicated dermal corset made of flexible collagenous fibers arranged as a helical network surrounding their body. The corset works as an outer skeleton, providing attachment for their swimming muscles and thus saving energy.[36] Depending on the position of these placoid scales on the body, they can be flexible and can be passively erected, allowing them to change their angle of attack. These scales also have riblets which are aligned in the direction of flow, these riblets reduce the drag force acting on the shark skin by pushing the vortex further away from the skin surface, inhibiting any high-velocity cross-stream flow.[37]
Scale morphology
[edit]The general anatomy of the scales varies, but all calcium composites hydrolize scales out side of main skeleton of them it's can be divided into three parts: the crown, the neck and the base. The scale pliability is related to the size of the base of the scale. The scales with higher flexibility have a smaller base, and thus are less rigidly attached to the stratum laxum. On the crown of the fast-swimming sharks there are a series of parallel riblets or ridges which run from an anterior to posterior direction.[38]
Analyzing the three components of the scale it can be concluded that the base of the denticle does not come into contact with any portion of the fluid flow.[39] The crown and the neck of the denticles however play a key role and are responsible for creating the turbulent vortices and eddies found near the skin's surface.[39] Because denticles come in so many different shapes and sizes, it can be expected that not all shapes will produce the same type of turbulent flow. During a recent research experiment biomimetic samples of shark denticles with a crescent like microstructure were tested in a water tank using a traction table as a slide. The experiment showed that the surface with denticles experienced a 10% drag reduction overall versus the smooth sample. The reason for this drag reduction was that the turbulent vortices became trapped between the denticles, creating a ‘cushion like’ barrier against the laminar flow.[40] This same type of experiment was performed by another research group which implemented more variation in their biomimetic sample. The second group arrived at the same conclusion as the first. However, because their experiment contained more variation within the samples they were able to achieve a high degree of experimental accuracy. In conclusion, they stated that more practical shapes were more durable than ones with intricate ridge-lines. The practical shapes were low profile and contained trapezoidal or semi-circular trough-like cross sections, and were less effective but nonetheless reduced drag by 6 or 7%.[41]
Drag reduction
[edit]

Sharks decrease drag and overall cost of transport (COT) through multiple different avenues. Pressure drag is created from the pressure difference between the anterior and posterior sides of the shark due to the amount of volume that is pushed past the shark to propel itself forward.[42] This type of drag is also directly proportional to the laminar flow. When the laminar flow increases around the fish the pressure drag does as well.[43] Frictional drag is a result of the interaction between the fluid against the shark's skin and can vary depending on how the boundary layer changes against the surface of the fish.[42]
The riblets impede the cross-stream translation of the streamwise vortices in the viscous sublayer. The mechanism is complex and not yet understood fully. Basically, the riblets inhibit the vortex formation near the surface because the vortex cannot fit in the valleys formed by the riblets. This pushes the vortex further up from the surface, interacting only with the riblet tips, not causing any high-velocity flow in the valleys. Since this high-velocity flow now only interacts with the riblet-tip, which is a very small surface area, the momentum transfer which causes drag is now much lower than before, thereby effectively reducing drag. Also, this reduces the cross-stream velocity fluctuations, which aids in momentum transfer too.[38]
Recent research has shown that there is a pre and post-breakdown regime in the near-wall boundary layer where the sublayer thickens at a declining rate and then abruptly undergoes a breakdown into turbulent vortices before finally collapsing. This system is completely self-regulating and mediates the growth and decay cycle; the vortices accumulate during the growth period and are abruptly liquidated into Strouhal arrays of hairpin vortices lifting off the wall. Lifting vortices are what push the boundary layer out and away from the surface of the shark which results in reducing the overall drag experienced by the fish.[44]
Technical application
[edit]The rough, sandpaper-like texture of shark and ray skin, coupled with its toughness, has led it to be valued as a source of rawhide leather, called shagreen. One of the many historical applications of shark shagreen was in making hand-grips for swords. The rough texture of the skin is also used in Japanese cuisine to make graters called oroshiki, by attaching pieces of shark skin to wooden boards. The small size of the scales grates the food very finely.

In the marine industry, fouling is the process by which something in the water becomes encrusted with sea life such as barnacles and algae. When ships' hulls are fouled, they are much less efficient (because they are rougher), and they are expensive and time-consuming to clean. Therefore, inexpensive and environmentally safe anti-fouling surfaces are in very high demand to increase the efficiency of shipping, fishing, and naval fleets, among other applications. Dermal denticles are a promising area of research for this type of application due to the fact that sharks are among the only fish without build up or growth on their scales. Studies by the U.S. Navy have shown that if a biomimetic material can be engineered, it could potentially lead to fuel cost savings for military vessels of up to 45%.[45]
There are many examples of biomimetic materials and surfaces based on the structure of aquatic organisms, including sharks. Such applications intend to enable more efficient movement through fluid mediums such as air, water, and oil.
Surfaces that mimic the skin of sharks have also been used in order to keep microorganisms and algae from coating the hulls of submarines and ships. One variety is traded as "sharklet".[46][47]
A lot of the new methods for replicating shark skin involve the use of polydimethylsiloxane (PDMS) for creating a mold. Usually the process involves taking a flat piece of shark skin, covering it with the PDMS to form a mold and pouring PDMS into that mold again to get a shark skin replica. This method has been used to create a biomimetic surface which has superhydrophobic properties, exhibiting the lotus effect.[46] One study found that these biomimetic surfaces reduced drag by up to 9%,[37] while with flapping motion drag reduction reached 12.3%.[48]
Denticles also provide drag reduction on objects where the main form of drag is caused by turbulent flow at the surface. A large portion of the total drag on long objects with relatively flat sides usually comes from turbulence at the wall, so riblets will have an appreciable effect. Along with marine applications, the aerospace industry can benefit greatly from these biomimetic designs. Other applications include pipes, where they score the insides to a riblet-like roughness and have discovered a 5% drag reduction, and a few percent reduction is claimed with competitive swimwear.[49]
Parametric modeling has been done on shark denticles with a wide range of design variations such as low and high-profile vortex generators.[50] Through this method, the most thorough characterization has been completed for symmetrical two-dimensional riblets with sawtooth, scalloped and blade cross sections.[49] These biomimetic models were designed and analyzed to see the effects of applying the denticle-like structures to the wings of various airplanes. During the simulation, it was noted that the sample altered how the low and high angles of attack reacted. Both the geometry of the denticles and their arrangement have a profound effect on the aerodynamic response of the aerofoils. Out of both the low and high-profile samples tested, the low-profile vortex generators outperformed the current smooth wing structures by 323%. This increase in performance is due to a separation bubble in the denticle's wake and stream-wise vortices that replenish momentum lost in the boundary layer due to skin friction.[50]
Scutes
[edit]
Scutes are similar to scales and serve the same function. Unlike the scales of fish, which are formed from the epidermis, scutes are formed in the lower vascular layer of the skin and the epidermal element is only the top surface. Forming in the living dermis, the scutes produce a horny outer layer, that is superficially similar to that of scales.
Scute comes from Latin for shield, and can take the form of:
- an external shield-like bony plate, or
- a modified, thickened scale that often is keeled or spiny, or
- a projecting, modified (rough and strongly ridged) scale, usually associated with the lateral line, or on the caudal peduncle forming caudal keels, or along the ventral profile.
Some fish, such as pineconefish, are completely or partially covered in scutes. River herrings and threadfins have an abdominal row of scutes, which are scales with raised, sharp points that are used for protection. Some jacks have a row of scutes following the lateral line on either side.
Scale development
[edit]Scales typically appear late in the development of fish. In the case of zebrafish, it takes 30 days after fertilization before the different layers needed to start forming the scales have differentiated and become organized. For this it is necessary that consolidation of the mesenchyme occurs, then morphogenesis is induced, and finally the process of differentiation or late metamorphosis occurs.[51][52]
- Mesenchyme consolidation: The consolidation or structuring of the mesenchyme originates during the development of the dermis. This process depends on whether the fish is cartilaginous or bony. For cartilaginous fish the structuring originates through the formation of two layers. The first is superficial and wide and the second is thin and compact. These two layers are separated by mesenchymal cells. Bony fish generate an acellular substrate organized by perpendicularly by collagen fibers. Subsequently, for both fish the fibroblasts elongate. These penetrate the compact layer of the mesenchyme, which consolidates prior to the formation of the scale, in order to initiate the dermal plate.[51][52][53]
- Morphogenesis induction: The morphogenesis is due to the formation of the epidermal papilla, which is generated by joining the epidermis and dermis through a process of invagination. Morphogenesis begins at the time when fibroblasts are relocated to the upper part of the compact mesenchyme. Throughout this process, the basal cells of the epithelium form a delimiting layer, which is located in the upper part of the mesenchyme. Subsequently, these cells will differentiate in the area where the scale primordium will arise.[51][52][53]
- Differentiation or late metamorphosis: This differentiation is generated by two different forms according to the type of scale being formed. The formation of elasmoid scales (cycloids and ctenoids) occurs through the formation of a space between the matrix of the epidermal papilla. This space contains collagen fibers. Around this space elasmoblasts differentiate and are responsible for generating the necessary material for the formation of the scale. Subsequently, matrix mineralization occurs, allowing the scale to acquire the rigid characteristic that identifies them.[51][52][53]
Unlike elasmoid scales, ganoid scales are composed of mineralized and non-mineralized collagen in different regions. The formation of these occurs through the entry of the surface cells of the mesenchyme into the matrix, the latter is composed of collagen fibers and is located around the vascular capillaries, thus giving rise to vascular cavities. At this point, elasmoblasts are replaced by osteoblasts, thus forming bone. The patches of the matrix of the scale that are not ossified are composed of compacted collagen that allow it to maintain the union with the mesenchyme. This are known as Sharpey fibers.[51][52][53]
One of the genes that regulate the development of scale formation in fish is the sonic hedgehog (shh) gene, which by means of the (shh) protein, involved in organogenesis and in the process of cellular communication, enable the formation of the scales.[54][55] The apolipoprotein E (ApoE), that allows the transport and metabolism of triglycerides and cholesterol, has an interaction with shh, because ApoE provides cholesterol to the shh signaling pathway. It has been shown that during the process of cell differentiation and interaction, the level of ApoE transcription is high, which has led to the conclusion that this protein is important for the late development of scales.[54][55]
Modified scales
[edit]Different groups of fish have evolved a number of modified scales to serve various functions.
- Almost all fishes have a lateral line, a system of mechanoreceptors that detect water movements. In bony fishes, the scales along the lateral line have central pores that allow water to contact the sensory cells.
- The dorsal fin spines of dogfish sharks and chimaeras, the stinging tail spines of stingrays, and the "saw" teeth of sawfishes and sawsharks are fused and modified placoid scales.
- Surgeonfish have a scalpel-like blade, which is a modified scale, on either side of the caudal peduncle.[56]
- Some herrings, anchovies, and halfbeaks have deciduous scales, which are easily shed and aid in escaping predators.
- Male Percina darters have a row of enlarged caducous scales between the pelvic fins and the anus.
- Porcupine fishes have scales modified into large external spines.
- By contrast, pufferfish have thinner, more hidden spines than porcupine fish, which become visible only when the fish puffs up. Unlike the porcupine fish, these spines are not modified scales, but develop under the control of the same network of genes that produce feathers and hairs in other vertebrates.[57][58]
-
Porcupine fish have scales modified into spines.
-
Pufferfish spines are not modified scales but are developed by an independent gene network.
Fish without scales
[edit]-
Mandarinfish lack scales and protect themselves with a layer of smelly and bitter slime.
Fish without scales usually evolve alternatives to the protection scales can provide, such as tough leathery skin or bony plates.
- Jawless fish (lampreys and hagfishes) have smooth skin without scales and without dermal bone.[59] Lampreys get some protection from a tough leathery skin. Hagfish exude copious quantities of slime or mucus if they are threatened.[60] They can tie themselves in an overhand knot, scraping off the slime as they go and freeing themselves from a predator.[61]
- Most eels are scaleless, though some species are covered with tiny smooth cycloid scales.
- Most catfish lack scales, though several families have body armour in the form of dermal plates or some sort of scute.[62]
- Mandarinfish lack scales and have a layer of smelly and bitter slime which blocks out disease and probably discourages predators, implying their bright coloration is aposematic.[63]
- Anglerfish have loose, thin skin often covered with fine forked dermal prickles or tubercles, but they do not have regular scales. They rely on camouflage to avoid the attention of predators, while their loose skin makes it difficult for predators to grab them.
Many groups of bony fishes, including pipefish, seahorses, boxfish, poachers, and several families of sticklebacks, have developed external bony plates, structurally resembling placoid scales, as protective armour against predators.
- Seahorses lack scales but have thin skin stretched over a bony plate armour arranged in rings through the length of their bodies.
- In boxfish, the plates fuse together to form a rigid shell or exoskeleton enclosing the entire body. These bony plates are not modified scales but skin that has been ossified. Because of this heavy armour boxfish are limited to slow movements, but few other fish are able to eat the adults.

| |
Eels seem scaleless, but some species are covered with tiny smooth cycloid scales.
| |
-
Boxfish have plates of ossified skin fused together to form a rigid shell.
-
Seahorses have thin skin stretched over bony plates arranged in rings.
Some fish, such as hoki and swordfish, are born with scales but shed them as they grow.
Filefish have rough non-overlapping scales with small spikes, which is why they are called filefish. Some filefish appear scaleless because their scales are so small.
Prominent scaling appears on tuna only along the lateral line and in the corselet, a protective band of thickened and enlarged scales in the shoulder region. Over most of their body tuna have scales so small that to casual inspection they seems scaleless.[64]
-
Some filefish appear scaleless because their scales are so small.
-
To casual examination tuna seem largely free of scales, but they are not.
Leviticus
[edit]A passage in Leviticus declares that, "of all that are in the waters... in the seas, and in the rivers" those that do not have both fins and scales "shall be an abomination unto you" and may not be eaten.[65] This eliminates all aquatic invertebrates as abominations and unclean, as well as any fish that lack scales (there do not seem to be fish that lack fins).
According to the chok or divine decrees of the Torah and the Talmud, for a fish to be declared kosher, it must have scales and fins.[66] The definition of "scale" differs from the definitions presented in biology, in that the scales of a kosher fish must be visible to the eye, and can be easily removed from the skin either by hand or scaling knife.[66] According to the kosher certification agency of the Orthodox Union, a fish is kosher if the scales can be removed without tearing its skin.[67] Thus carp and salmon are kosher, whereas a shark, whose scales are microscopic, a sturgeon, whose scutes can not be easily removed without cutting them out of the body, are all not kosher. Other non-kosher fish include catfish, eels, freshwater cod, snake mackerels, and puffer fish.[66]
Lepidophagy
[edit]
Lepidophagy (Ancient Greek for scale-eating) is a specialised feeding behaviour in fish that involves eating the scales of other fish.[69] Lepidophagy has independently evolved in at least five freshwater families and seven marine families.[70]
Fish scales can be nutritious, containing a dermal portion and a layer of protein-rich mucus apart from the layers of keratin and enamel. They are a rich source of calcium phosphate.[70] However, the energy expended to make a strike versus the amount of scales consumed per strike puts a limit on the size of lepidophagous fish, and they are usually much smaller than their prey.[70] Scale eating behaviour usually evolves because of lack of food and extreme environmental conditions. The eating of scales and the skin surrounding the scales provides protein rich nutrients that may not be available elsewhere in the niche.[71]
Fish jaws normally show bilateral symmetry. An exception occurs with the scale-eating cichlid Perissodus microlepis. The jaws of this fish occur in two distinct morphological forms. One morph has its jaw twisted to the left, allowing it to eat scales more readily on its victim's right flank. The other morph has its jaw twisted to the right, which makes it easier to eat scales on its victim's left flank. The relative abundance of the two morphs in populations is regulated by frequency-dependent selection.[68][72][73]
See also
[edit]- Age determination in fish
- Animal coloration
- Animal reflectors
- Photonic crystals
- Reptile scale
- Scale (zoology)
- Scale armour
- Snake scales
- Urokotori – Japanese fish scaler
References
[edit]- ^ Scale Etymonline. Retrieved 28 April 2019.
- ^ Mongera, A.; Nüsslein-Volhard, C. (2013). "Scales of fish arise from mesoderm". Current Biology. 23 (9): R338 – R339. Bibcode:2013CBio...23.R338M. doi:10.1016/j.cub.2013.02.056. PMID 23660349.
- ^ Sharpe, P. T. (2001). "Fish scale development: Hair today, teeth and scales yesterday?". Current Biology. 11 (18): R751 – R752. Bibcode:2001CBio...11.R751S. doi:10.1016/S0960-9822(01)00438-9. PMID 11566120. S2CID 18868124.
- ^ Turner, S.; Tarling, D. H. (1982). "Thelodont and other agnathan distributions as tests of Lower Paleozoic continental reconstructions". Palaeogeography, Palaeoclimatology, Palaeoecology. 39 (3–4): 295–311. Bibcode:1982PPP....39..295T. doi:10.1016/0031-0182(82)90027-X.
- ^ Märss, T. (2006). "Exoskeletal ultrasculpture of early vertebrates". Journal of Vertebrate Paleontology. 26 (2): 235–252. doi:10.1671/0272-4634(2006)26[235:EUOEV]2.0.CO;2. S2CID 85993241.
- ^ a b Janvier, Philippe (1998). "Early vertebrates and their extant relatives". Early Vertebrates. Oxford University Press. pp. 123–127. ISBN 978-0-19-854047-2.
- ^ a b Turner, S. (1991). "Monophyly and interrelationships of the Thelodonti". In M. M. Chang; Y. H. Liu; G. R. Zhang (eds.). Early Vertebrates and Related Problems of Evolutionary Biology. Science Press, Beijing. pp. 87–119.
- ^ Märss, T. (1986). "Squamation of the thelodont agnathan Phlebolepis". Journal of Vertebrate Paleontology. 6 (1): 1–11. Bibcode:1986JVPal...6....1M. doi:10.1080/02724634.1986.10011593.
- ^ Botella, H.; J. I. Valenzuela-Rios; P. Carls (2006). "A New Early Devonian thelodont from Celtiberia (Spain), with a revision of Spanish thelodonts". Palaeontology. 49 (1): 141–154. Bibcode:2006Palgy..49..141B. doi:10.1111/j.1475-4983.2005.00534.x. S2CID 128939911.
- ^ Ferrón, Humberto G.; Botella, Héctor (2017). "Squamation and ecology of thelodonts". PLOS ONE. 12 (2): e0172781. Bibcode:2017PLoSO..1272781F. doi:10.1371/journal.pone.0172781. PMC 5328365. PMID 28241029.
- ^ MICHAEL ALLABY "cosmoid scale ." A Dictionary of Zoology . . Encyclopedia.com. 29 Oct. 2019 <https://www.encyclopedia.com>
- ^ Zylberberg, L., Meunier, F.J., Laurin, M. (2010). A microanatomical and histological study of the postcranial dermal skeleton in the Devonian sarcopterygian Eusthenopteron foordi, Acta Palaeontologica Polonica 55: 459–470.
- ^ Bergen, Dylan J. M.; Kague, Erika; Hammond, Chrissy L. (2019). "Zebrafish as an Emerging Model for Osteoporosis: A Primary Testing Platform for Screening New Osteo-Active Compounds". Frontiers in Endocrinology. 10: 6. doi:10.3389/fendo.2019.00006. ISSN 1664-2392. PMC 6361756. PMID 30761080.
- ^ de Vrieze, E.; van Kessel, M. A. H. J.; Peters, H. M.; Spanings, F. A. T.; Flik, G.; Metz, J. R. (1 February 2014). "Prednisolone induces osteoporosis-like phenotype in regenerating zebrafish scales". Osteoporosis International. 25 (2): 567–578. doi:10.1007/s00198-013-2441-3. hdl:2066/123472. ISSN 1433-2965. PMID 23903952. S2CID 21829206.
- ^ a b Zylberberg, L.; Sire, J. -Y.; Nanci, A. (1997). "Immunodetection of amelogenin-like proteins in the ganoine of experimentally regenerating scales of Calamoichthys calabaricus, a primitive actinopterygian fish". The Anatomical Record. 249 (1): 86–95. doi:10.1002/(SICI)1097-0185(199709)249:1<86::AID-AR11>3.0.CO;2-X. PMID 9294653.
- ^ Sire, Jean-Yves; Donoghue, Philip C. J.; Vickaryous, Matthews K. (2009). "Origin and evolution of the integumentary skeleton in non-tetrapod vertebrates". Journal of Anatomy. 214 (4): 409–440. doi:10.1111/j.1469-7580.2009.01046.x. ISSN 0021-8782. PMC 2736117. PMID 19422423.
- ^ a b c Richter, M. (1995). "A microstructural study of the ganoine tissue of selected lower vertebrates". Zoological Journal of the Linnean Society. 114 (2): 173–212. doi:10.1006/zjls.1995.0023.
- ^ a b Bruet, B. J. F.; Song, J.; Boyce, M. C.; Ortiz, C. (2008). "Materials design principles of ancient fish armour". Nature Materials. 7 (9): 748–756. Bibcode:2008NatMa...7..748B. doi:10.1038/nmat2231. PMID 18660814.
- ^ a b Sherman, Vincent R.; Yaraghi, Nicholas A.; Kisailus, David; Meyers, Marc A. (1 December 2016). "Microstructural and geometric influences in the protective scales of Atractosteus spatula". Journal of the Royal Society Interface. 13 (125): 20160595. doi:10.1098/rsif.2016.0595. ISSN 1742-5689. PMC 5221522. PMID 27974575.
- ^ "Missouri Alligator Gar Management and Restoration Plan" (PDF). Missouri Department of Conservation Fisheries Division. January 22, 2013. Archived from the original (PDF) on May 6, 2016. Retrieved April 12, 2019.
- ^ Lagler, K. F., J. E. Bardach, and R. R. Miller (1962) Ichthyology. New York: John Wiley & Sons.
- ^ Ballard, Bonnie; Cheek, Ryan (2 July 2016). Exotic Animal Medicine for the Veterinary Technician. John Wiley & Sons. ISBN 978-1-118-92421-1.
- ^ McGrouther, Mark (2 December 2019). "Cycloid and Ctenoid Scales". The Australian Museum. Retrieved 29 December 2021.
- ^ Pouyaud, L.; Sudarto, Guy G. Teugels (2003). "The different colour varieties of the Asian arowana Scleropages formosus (Osteoglossidae) are distinct species: morphologic and genetic evidences". Cybium. 27 (4): 287–305.
- ^ Ismail, M. (1989). Systematics, Zoogeography, and Conservation of the Freshwater Fishes of Peninsular Malaysia (Doctoral Dissertation ed.). Colorado State University.
- ^ E.J. Brill (1953). The Fishes of the Indo-Australian Archipelago. E.J. Brill. pp. 306–307.
- ^ a b c d Kawasaki, Kenta C., "A Genetic Analysis of Cichlid Scale Morphology" (2016). Masters Theses May 2014 - current. 425. http://scholarworks.umass.edu/masters_theses_2/425
- ^ Helfman, Gene (2009). The Diversity of Fishes Biology, Evolution, and Ecology. Wiley-Blackwell.
- ^ a b c Herring, Peter (2002). The Biology of the Deep Ocean. Oxford: Oxford University Press. pp. 193–195. ISBN 9780198549567.
- ^ "There Are Probably Fish Scales In Your Lipstick". HuffPost India. 23 April 2015. Retrieved 6 May 2019.
- ^ Martin, R. Aidan. "Skin of the Teeth". Retrieved 28 August 2007.
- ^ Fürstner, Reiner; Barthlott, Wilhelm; Neinhuis, Christoph; Walzel, Peter (1 February 2005). "Wetting and Self-Cleaning Properties of Artificial Superhydrophobic Surfaces". Langmuir. 21 (3): 956–961. doi:10.1021/la0401011. ISSN 0743-7463. PMID 15667174.
- ^ Lauder, George V.; Wainwright, Dylan K.; Domel, August G.; Weaver, James C.; Wen, Li; Bertoldi, Katia (2016). "Structure, biomimetics, and fluid dynamics of fish skin surfaces". Physical Review Fluids. 1 (6): 060502. Bibcode:2016PhRvF...1f0502L. doi:10.1103/PhysRevFluids.1.060502. S2CID 18118663.
- ^ a b Feld, Katrine; Kolborg, Anne Noer; Nyborg, Camilla Marie; Salewski, Mirko; Steffensen, John Fleng; Berg-Sørensen, Kirstine (24 May 2019). "Dermal Denticles of Three Slowly Swimming Shark Species: Microscopy and Flow Visualization". Biomimetics. 4 (2): 38. doi:10.3390/biomimetics4020038. ISSN 2313-7673. PMC 6631580. PMID 31137624.
- ^ Fletcher, Thomas; Altringham, John; Peakall, Jeffrey; Wignall, Paul; Dorrell, Robert (7 August 2014). "Hydrodynamics of fossil fishes". Proceedings of the Royal Society B: Biological Sciences. 281 (1788): 20140703. doi:10.1098/rspb.2014.0703. ISSN 0962-8452. PMC 4083790. PMID 24943377.
- ^ Martin, R. Aidan. "The Importance of Being Cartilaginous". ReefQuest Centre for Shark Research. Retrieved 29 August 2009.
- ^ a b Hage, W.; Bruse, M.; Bechert, D. W. (1 May 2000). "Experiments with three-dimensional riblets as an idealized model of shark skin". Experiments in Fluids. 28 (5): 403–412. Bibcode:2000ExFl...28..403B. doi:10.1007/s003480050400. ISSN 1432-1114. S2CID 122574419.
- ^ a b Motta, Philip; Habegger, Maria Laura; Lang, Amy; Hueter, Robert; Davis, Jessica (1 October 2012). "Scale morphology and flexibility in the shortfin mako Isurus oxyrinchus and the blacktip shark Carcharhinus limbatus". Journal of Morphology. 273 (10): 1096–1110. doi:10.1002/jmor.20047. ISSN 1097-4687. PMID 22730019. S2CID 23881820.
- ^ a b Dou, Zhaoliang; Wang, Jiadao; Chen, Darong (1 December 2012). "Bionic Research on Fish Scales for Drag Reduction". Journal of Bionic Engineering. 9 (4): 457–464. doi:10.1016/S1672-6529(11)60140-6. ISSN 1672-6529. S2CID 137143652.
- ^ "Experimental investigations on drag-reduction characteristics of bionic surface with water-trapping microstructures of fish scales" (PDF).
- ^ Palmer, Colin; Young, Mark T. (14 January 2015). "Surface drag reduction and flow separation control in pelagic vertebrates, with implications for interpreting scale morphologies in fossil taxa". Royal Society Open Science. 2 (1): 140163. Bibcode:2015RSOS....240163P. doi:10.1098/rsos.140163. ISSN 2054-5703. PMC 4448786. PMID 26064576.
- ^ a b Lauder, George V.; Wainwright, Dylan K.; Domel, August G.; Weaver, James C.; Wen, Li; Bertoldi, Katia (18 October 2016). "Structure, biomimetics, and fluid dynamics of fish skin surfaces". Physical Review Fluids. 1 (6): 060502. Bibcode:2016PhRvF...1f0502L. doi:10.1103/PhysRevFluids.1.060502.
- ^ Muthuramalingam, Muthukumar; Villemin, Leo S.; Bruecker, Christoph (29 April 2019). "Streak formation in flow over Biomimetic Fish Scale Arrays". The Journal of Experimental Biology. 222 (Pt 16): jeb205963. arXiv:1904.12752. Bibcode:2019arXiv190412752M. doi:10.1242/jeb.205963. PMID 31375542. S2CID 139103148.
- ^ Bandyopadhyay, Promode R.; Hellum, Aren M. (23 October 2014). "Modeling how shark and dolphin skin patterns control transitional wall-turbulence vorticity patterns using spatiotemporal phase reset mechanisms". Scientific Reports. 4: 6650. Bibcode:2014NatSR...4.6650B. doi:10.1038/srep06650. ISSN 2045-2322. PMC 4206846. PMID 25338940.
- ^ Magin, Chelsea M.; Cooper, Scott P.; Brennan, Anthony B. (1 April 2010). "Non-toxic antifouling strategies". Materials Today. 13 (4): 36–44. doi:10.1016/S1369-7021(10)70058-4. ISSN 1369-7021.
- ^ a b Liu, Yunhong; Li, Guangji (15 December 2012). "A new method for producing "Lotus Effect" on a biomimetic shark skin". Journal of Colloid and Interface Science. 388 (1): 235–242. Bibcode:2012JCIS..388..235L. doi:10.1016/j.jcis.2012.08.033. ISSN 0021-9797. PMID 22995249.
- ^ "Sharklet Discovery | Sharklet Technologies, Inc". www.sharklet.com. Retrieved 26 September 2018.
- ^ Lauder, George V.; Oeffner, Johannes (1 March 2012). "The hydrodynamic function of shark skin and two biomimetic applications". Journal of Experimental Biology. 215 (5): 785–795. Bibcode:2012JExpB.215..785O. doi:10.1242/jeb.063040. ISSN 1477-9145. PMID 22323201.
- ^ a b Dean, Brian & Bhushan, Bharat. (2010). Shark-Skin Surfaces for Fluid-Drag Reduction in Turbulent Flow: A Review. Philosophical transactions. Series A, Mathematical, physical, and engineering sciences. 368. 4775-806. 10.1098/rsta.2010.0201.
- ^ a b Domel, August G.; Saadat, Mehdi; Weaver, James C.; Haj-Hariri, Hossein; Bertoldi, Katia; Lauder, George V. (28 February 2018). "Shark skin-inspired designs that improve aerodynamic performance". Journal of the Royal Society Interface. 15 (139): 20170828. doi:10.1098/rsif.2017.0828. PMC 5832729. PMID 29436512.
- ^ a b c d e Sire, J.Y.; Huysseune, A.N.N. (2003). "Formation of dermal skeletal and dental tissues in fish: a comparative and evolutionary approach". Biological Reviews. 78 (2): 219–249. doi:10.1017/S1464793102006073. PMID 12803422. S2CID 19556201.
- ^ a b c d e Le Guellec, D.; Morvan-Dubois, G.; Sire, J.Y. (2004). "Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio)". International Journal of Developmental Biology. 48 (2–3): 217–231. doi:10.1387/ijdb.15272388. PMID 15272388.
- ^ a b c d Sire, J.Y. (2001). "Teeth outside the mouth in teleost fishes: how to benefit from a developmental accident". Evolution & Development. 3 (2): 104–108. doi:10.1046/j.1525-142x.2001.003002104.x. PMID 11341672. S2CID 13353402.
- ^ a b Sire, J.Y.; Akimenko, M.A. (2003). "Scale development in fish: a review, with description of sonic hedgehog (shh) expression in the zebrafish (Danio rerio)". International Journal of Developmental Biology. 48 (2–3): 233–247. doi:10.1387/ijdb.15272389. PMID 15272389.
- ^ a b Monnot, M.J.; Babin, P.J.; Poleo, G.; Andre, M.; Laforest, L.; Ballagny, C.; Akimenko, M.A. (1999). "Epidermal expression of apolipoprotein E gene during fin and scale development and fin regeneration in zebrafish". Developmental Dynamics. 214 (3): 207–215. doi:10.1002/(SICI)1097-0177(199903)214:3<207::AID-AJA4>3.0.CO;2-5. PMID 10090147.
- ^ Sorenson, L.; Santini, F.; Carnevale, G.; Alfaro, M.E. (2013). "A multi-locus timetree of surgeonfishes (Acanthuridae, Percomorpha), with revised family taxonomy". Molecular Phylogenetics & Evolution. 68 (1): 150–160. Bibcode:2013MolPE..68..150S. doi:10.1016/j.ympev.2013.03.014. PMID 23542000.
- ^ How the pufferfish got its wacky spines Phys.org, 25 July 2019.
- ^ Shono, T.; Thiery, A.P.; Cooper, R.L.; Kurokawa, D.; Britz, R.; Okabe, M.; Fraser, G.J. (2019). "Evolution and Developmental Diversity of Skin Spines in Pufferfishes". iScience. 19: 1248–1259. Bibcode:2019iSci...19.1248S. doi:10.1016/j.isci.2019.06.003. PMC 6831732. PMID 31353167.
- ^ Coolidge E, Hedrick MS and Milsom WK (2011) "Ventilatory Systems". In: McKenzie DJ, Farrell AP and Brauner CJ (Eds) Fish Physiology: Primitive Fishes, Elsevier, Page 182–213. ISBN 9780080549521
- ^ Rothschild, Anna (1 April 2013). "Hagfish slime: The clothing of the future?". BBC News. Retrieved 2 April 2013.
- ^ Yong, Ed (23 January 2019). "No One Is Prepared for Hagfish Slime". The Atlantic. Retrieved 26 January 2019.
- ^ Friel, J P; Lundberg, J G (1996). "Micromyzon akamai, gen. et sp. nov., a small and eyeless banjo catfish (Siluriformes: Aspredinidae) from the river channels of the lower Amazon basin". Copeia. 1996 (3): 641–648. doi:10.2307/1447528. JSTOR 1447528.
- ^ Sadovy, Y.; Randall, J. E.; Rasotto, Maria B. (May 2005). "Skin structure in six dragonet species (Gobiesociformes; Callionymidae): Interspecific differences in glandular cell types and mucus secretion". Journal of Fish Biology. 66 (5): 1411–1418. Bibcode:2005JFBio..66.1411S. doi:10.1111/j.0022-1112.2005.00692.x.
- ^ Do tunas have scales? Northeast Fisheries Science Center, NOAA Fisheries. Accessed 4 August 2019.
- ^ Leviticus 11:9–10
- ^ a b c Aryeh Citron, "All About Kosher Fish"
- ^ Verifying Kosher Fish OU Kosher Certification. Retrieved 9 August 2019.
- ^ a b Lee, H. J.; Kusche, H.; Meyer, A. (2012). "Handed Foraging Behavior in Scale-Eating Cichlid Fish: Its Potential Role in Shaping Morphological Asymmetry". PLOS ONE. 7 (9): e44670. Bibcode:2012PLoSO...744670L. doi:10.1371/journal.pone.0044670. PMC 3435272. PMID 22970282.
- ^ Froese, R. and D. Pauly. Editors. "Glossary: Lepidophagy". FishBase. Retrieved 12 April 2007.
{{cite web}}:|author=has generic name (help) - ^ a b c Janovetz, Jeff (2005). "Functional morphology of feeding in the scale-eating specialist Catoprion mento" (PDF). The Journal of Experimental Biology. 208 (Pt 24): 4757–4768. Bibcode:2005JExpB.208.4757J. doi:10.1242/jeb.01938. PMID 16326957. S2CID 15566769.
- ^ Martin, C.; P.C. Wainwright (2011). "Trophic novelty is linked to exceptional rates of morphological diversification in two adaptive radiations of Cyprinodon pupfish". Evolution. 65 (8): 2197–2212. doi:10.1111/j.1558-5646.2011.01294.x. PMID 21790569. S2CID 23695342.
- ^ Hori, M. (1993). "Frequency-dependent natural selection in the handedness of scale-eating cichlid fish". Science. 260 (5105): 216–219. Bibcode:1993Sci...260..216H. doi:10.1126/science.260.5105.216. PMID 17807183. S2CID 33113282.
- ^ Stewart, T. A.; Albertson, R. C. (2010). "Evolution of a unique predatory feeding apparatus: functional anatomy, development and a genetic locus for jaw laterality in Lake Tanganyika scale-eating cichlids". BMC Biology. 8 (1): 8. doi:10.1186/1741-7007-8-8. PMC 2828976. PMID 20102595.
Further reading
[edit]- Helfman, G.S., B.B. Collette and D.E. Facey (1997). The Diversity of Fishes. Blackwell Science. pp. 33–36. ISBN 978-0-86542-256-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Schultze, H.P. (2016). "Scales, enamel, cosmine, ganoine, and early osteichthyans". Comptes Rendus Palevol. 15 (1–2): 83–102. Bibcode:2016CRPal..15...83S. doi:10.1016/j.crpv.2015.04.001.

![The alligator gar has a tough armouring of rhomboidal-shaped ganoid scales.[19]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/bd/Alligator_gar_fish.jpg/395px-Alligator_gar_fish.jpg)