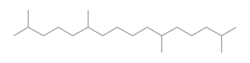
Crocetane
 | |
| Names | |
|---|---|
| IUPAC name
2,6,11,15-Tetramethylhexadecane[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H42 | |
| Molar mass | 282.556 g·mol−1 |
| Related compounds | |
Related alkanes
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Crocetane, or 2,6,11,15-tetramethylhexadecane, is an isoprenoid hydrocarbon compound. Unlike its isomer phytane, crocetane has a tail-to-tail linked isoprenoid skeleton. Crocetane has been detected in modern sediments and geological records as a biomarker, often associated with anaerobic methane oxidation.
Research
[edit]Crocetane was first studied[2] in the late 1920s and early 1930s for the structural identification of crocetin, which is its polyunsaturated diacid analogue. The infrared spectrum was reported in 1950,[3] the mass spectrum was described in 1968[4] and the 1H and 13C NMR spectra was obtained in 1990s.[2]
In 1994, Liangqiao Bian[5] first reported strong 13C depletion in crocetane from anoxic sediments in the Kattegat. Such low 13C content is thought to originate from microbes harvesting biogenic methane, which is always 13C depleted,[6] as a carbon source. Years later several groups[7][8][9] made similar observations in either modern or ancient sediments near methane seeps. Crocetane was found in environments with anaerobic methane oxidizing consortium, composed of methanotrophic archea and sulfate-reducing bacteria. These work makes crocetane the first biomarker[10] of anaerobic methanotrophy.
In 2009, Ercin Maslen and her colleagues detected crocetane in highly-mature Devonian sediments and crude oils of the Western Canada Sedimentary Basin.[11] They propose that natural product precursor for this crocetane is green sulfur bacteria derived isorenieratene and palaerernieratene, which means that crocetane can also be related to photic zone euxinia in highly matured samples.
Analysis
[edit]Due to structural similarities, crocetane often co-elutes with phytane and is hard to identify.[12] People have been using specialized gas chromatographic methods to achieve partial separation. For example, Volker Thiel and his colleagues used a 25-m squalene capillary column with hydrogen as a carrier gas.[7]
For the same reason mass spectra of crocetane and phytane are very similar except for that crocetane does not have intense m/z=183 fragments.[12] To identify crocetane, the mass spectrometer can be operated in selection ion monitoring(SIM) mode to monitor m/z 113, 169, 183, 197 and 282.[11] Paul Greenwood and Roger Summons in 2003 reported using GC MS-MS instrument to measure the daughter ion of m/z 196→127/126 and 168→126 to distinguish crocetane from phytane.[13]
References
[edit]- ^ "Hexadecane, 2,6,11,15-tetramethyl-". webbook.nist.gov.
- ^ a b Robson, J. N.; Rowland, S. J. (1993-09-01). "Synthesis, chromatographic and spectral characterisation of 2,6,11,15-tetramethylhexadecane (crocetane) and 2,6,9,13-tetramethyltetradecane: reference acyclic isoprenoids for geochemical studies". Organic Geochemistry. 20 (7): 1093–1098. Bibcode:1993OrGeo..20.1093R. doi:10.1016/0146-6380(93)90117-T.
- ^ Pliva, Josef; Sorensen, Andreas (1950). "Studies Related to Pristane: IV. InfraRed Spectra" (PDF). Acta Chemica Scandinavica. 4: 846–849. doi:10.3891/acta.chem.scand.04-0846.
- ^ McCarthy, E. D.; Han, Jerry; Calvin, Melvin (1968-08-01). "Hydrogen atom transfer in mass spectrometric fragmentation patterns of saturated aliphatic hydrocarbons". Analytical Chemistry. 40 (10): 1475–1480. doi:10.1021/ac60266a021. ISSN 0003-2700.
- ^ Bian, Liangqiao (1994). Isotopic biogeochemistry of individual compounds in a modern coastal marine sediment (Kattegat, Denmark and Sweden) (M.Sc. thesis). Dept. of Geological Sciences, Univ. Indiana.
- ^ Whiticar, Michael J. (1999-09-30). "Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane". Chemical Geology. 161 (1–3): 291–314. Bibcode:1999ChGeo.161..291W. doi:10.1016/S0009-2541(99)00092-3.
- ^ a b Thiel, Volker; Peckmann, Jörn; Seifert, Richard; Wehrung, Patrick; Reitner, Joachim; Michaelis, Walter (1999-12-01). "Highly isotopically depleted isoprenoids: molecular markers for ancient methane venting". Geochimica et Cosmochimica Acta. 63 (23–24): 3959–3966. Bibcode:1999GeCoA..63.3959T. doi:10.1016/S0016-7037(99)00177-5.
- ^ Hinrichs, Kai-Uwe; Summons, Roger E; Orphan, Victoria; Sylva, Sean P; Hayes, John M (2000-12-01). "Molecular and isotopic analysis of anaerobic methane-oxidizing communities in marine sediments". Organic Geochemistry. 31 (12): 1685–1701. Bibcode:2000OrGeo..31.1685H. doi:10.1016/S0146-6380(00)00106-6.
- ^ Elvert, Marcus; Suess, Erwin; Whiticar, Michael J. (1999). "Anaerobic methane oxidation associated with marine gas hydrates: superlight C-isotopes from saturated and unsaturated C20 and C25 irregular isoprenoids". Naturwissenschaften. 86 (6): 295–300. Bibcode:1999NW.....86..295E. doi:10.1007/s001140050619. ISSN 0028-1042. S2CID 31718134.
- ^ Hinrichs, K.-U.; Boetius, A. (2002-01-01). Wefer, Professor Dr Gerold; Billett, David; Hebbeln, Dierk; Jørgensen, Bo Barker; Schlüter, Michael; van Weering, Tjeerd C. E. (eds.). Ocean Margin Systems. Springer Berlin Heidelberg. pp. 457–477. doi:10.1007/978-3-662-05127-6_28. ISBN 9783642078729.
- ^ a b Maslen, Ercin; Grice, Kliti; Gale, Julian D.; Hallmann, Christian; Horsfield, Brian (2009-01-01). "Crocetane: A potential marker of photic zone euxinia in thermally mature sediments and crude oils of Devonian age". Organic Geochemistry. 40 (1): 1–11. Bibcode:2009OrGeo..40....1M. doi:10.1016/j.orggeochem.2008.10.005.
- ^ a b Peters, K. E.; Walters, C. C.; Moldowan, J. M. (2005). The Biomarker Guide, Volume 2. Cambridge University Press. pp. 509–510. ISBN 9780521781589.
- ^ Greenwood, Paul F.; Summons, Roger E. (2003-08-01). "GC–MS detection and significance of crocetane and pentamethylicosane in sediments and crude oils". Organic Geochemistry. 34 (8): 1211–1222. Bibcode:2003OrGeo..34.1211G. doi:10.1016/S0146-6380(03)00062-7.
