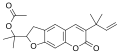
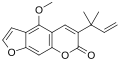
Chalepensin
 | |
| Names | |
|---|---|
| IUPAC name
6-(2-Methylbut-3-en-2-yl)furo[3,2-g]chromen-7-one
| |
| Other names
Xyloltenin; 3-(α,α-dimethylallyl)psoralen
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H14O3 | |
| Molar mass | 254.285 g·mol−1 |
| Melting point | 82–83 °C (180–181 °F; 355–356 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chalepensin is a chemical compound of the furanocoumarin class. Originally isolated in 1967 from fringed rue (Ruta chalepensis),[1] from which it derives its name, it has also been found in other plants of the genus Ruta including common rue (Ruta graveolens)[2] and mountain rue (Ruta montana).[3]
Chemical properties
[edit]Chalepensin forms colorless crystalline needles with a melting point of 82-83 °C.[4]
Research
[edit]Chalepensin has been shown to have antifertility effects in female rats.[2][5][6] This may be the result of toxic effects chalepensin has on the ovaries.[5] This antifertility effect may provide some scientific evidence in support of the traditional uses of fringed rue[6] and modern use of rue oil (oil from plants of the genus Ruta) in South America[7] as an abortifacient.
Chalepensis has also been shown to have antibacterial activity against Streptococcus mutans and methicillin-resistant Staphylococcus aureus (MRSA).[8]
Related compounds
[edit]Several chemical compounds that have the same core chemical structure as chalepensin are known, including chalepin, rutamarin, 5-methoxychalepensin, and 5,8-dimethoxychalepensin.
-
Chalepin
-
Rutamarin
-
5-Methoxychalepensin
-
5,8-Dimethoxychalepensin
References
[edit]- ^ Brooker, Robert M.; Eble, John N.; Starkovsky, Nicolas A. (1967). "Chalepensin, chalepin, and chalepin acetate, three novel furocoumarins from ruta chalepensis". Lloydia. 30 (1): 73–77.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Kong, Y.; Lau, C.; Wat, K.; Ng, K.; But, P.; Cheng, K.; Waterman, P. (1989). "Antifertility Principle of Ruta graveolens". Planta Medica. 55 (2): 176–178. doi:10.1055/s-2006-961917. PMID 2748734.
- ^ Touati, Driss; Atta-Ur-Rahman; Ulubelen, Ayhan (2000). "Alkaloids from Ruta montana". Phytochemistry. 53 (2): 277–279. doi:10.1016/S0031-9422(99)00486-0. PMID 10680183.
- ^ Wu, Tian-Shung; Shi, Li-Shian; Wang, Jhi-Joung; Iou, Song-Chou; Chang, Hsien-Chang; Chen, Yuh-Pan; Kuo, Yao-Haur; Chang, Ya-Ling; Tenge, Che-Ming (2003). "Cytotoxic and Antiplatelet Aggregation Principles of Ruta Graveolens". Journal of the Chinese Chemical Society. 50: 171–178. doi:10.1002/jccs.200300024.
- ^ a b Ulubelen, A.; Ertugrul, L.; Birman, H.; Yigit, R.; Erseven, G.; Olgac, V. (1994). "Antifertility effects of some coumarins isolated from Ruta chalepensis andR. Chalepensis var.latifolia in rodents". Phytotherapy Research. 8 (4): 233–236. doi:10.1002/ptr.2650080409. S2CID 85292230.
- ^ a b Nahar, Lutfun; Al-Majmaie, Shaymaa; Al-Groshi, Afaf; Rasul, Azhar; Sarker, Satyajit D. (2021). "Chalepin and Chalepensin: Occurrence, Biosynthesis and Therapeutic Potential". Molecules. 26 (6): 1609. doi:10.3390/molecules26061609. PMC 7999183. PMID 33799365.
- ^ Ciganda, Carmen; Laborde, Amalia (2003). "Herbal Infusions Used for Induced Abortion". Journal of Toxicology: Clinical Toxicology. 41 (3): 235–239. doi:10.1081/CLT-120021104. PMID 12807304. S2CID 44851492.
- ^ Al-Majmaie, Shaymaa; Nahar, Lutfun; Rahman, M. Mukhlesur; Nath, Sushmita; Saha, Priyanka; Talukdar, Anupam Das; Sharples, George P.; Sarker, Satyajit D. (2021). "Anti-MRSA Constituents from Ruta chalepensis (Rutaceae) Grown in Iraq, and in Silico Studies on Two of Most Active Compounds, Chalepensin and 6-Hydroxy-rutin 3′,7-Dimethyl ether". Molecules. 26 (4): 1114. doi:10.3390/molecules26041114. PMC 7923287. PMID 33669881.