Cefiderocol
 | |
| Clinical data | |
|---|---|
| Trade names | Fetroja, Fetcroja |
| Other names | RSC-649266 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620008 |
| License data | |
| Routes of administration | Intravenous infusion |
| Drug class | Siderophore cephalosporins |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 56–58%[4] |
| Elimination half-life | 2.8 hours |
| Excretion | mainly kidney (60–70% unchanged) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
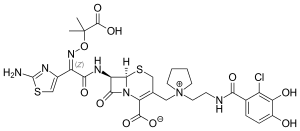
| Formula | C30H34ClN7O10S2 |
| Molar mass | 752.21 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cefiderocol, sold under the brand name Fetroja (by Shionogi) among others, is an antibiotic used to treat complicated urinary tract infections when no other options are available.[5] It is indicated for the treatment of multi-drug-resistant Gram-negative bacteria including Pseudomonas aeruginosa.[6][7][8] It is given by injection into a vein.[1]
Common side effects include diarrhea, infusion site reactions, constipation and rash.[9]
Cefiderocol is in the cephalosporin family of medications.[5][10] It was approved for medical use in the United States in November 2019, and in the European Union in April 2020.[5][11][2] In September 2020, cefiderocol (Fetroja) received FDA approval[12] as supplemental New Drug Application (sNDA) for treatment of hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) when caused by Gram-negative bacteria resistant to other antibiotics. It is on the World Health Organization's List of Essential Medicines.[13]
Medical uses
[edit]Cefiderocol is used to treat adults with complicated urinary tract infections, including kidney infections caused by susceptible Gram-negative microorganisms, who have limited or no alternative treatment options.[5][10]
In the United States, cefiderocol is indicated in adults 18 years of age or older who have limited or no alternative treatment options for the treatment of complicated urinary tract infections (cUTIs), including pyelonephritis caused by the following susceptible Gram-negative microorganisms: Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, and Enterobacter cloacae complex.[1]
For the treatment of severe pneumonia (HABP and VABP), it is indicated in patients 18 years of age and older whose pneumonia is not responding to other, more commonly used antibiotics and is confirmed to be caused by one of the following Gram-negative organisms:
- Acinetobacter baumannii complex
- Escherichia coli
- Enterobacter cloacae complex
- Klebsiella pneumoniae
- Pseudomonas aeruginosa
- Serratia marcescens
This indication is supported by a study in which cefiderocol was not inferior to meropenem in treating nosocomial pneumonia caused by Gram-negative bacteria. The primary endpoint of the study was mortality due to any cause at day 14, where both antibiotics were shown to be equally effective.[14]
Cefiderocol has been associated with the emergence of heteroresistance, especially in A. baumannii and P. aeruginosa.[15] In A. baumannii, the main mechanism identified so far involves mutations in the TonB-dependent receptor gene piuA.[16] In other Enterobacteriales, amplification of beta-lactamase genes, such as blaSHV, contributes to heteroresistance development.[17]
As of 2020, cefiderocol is indicated in the European Union for the treatment of infections due to aerobic Gram-negative bacteria in adults with limited treatment options.[2]
Adverse effects
[edit]Cefiderocol may cause serious and life-threatening allergic reactions, severe diarrhea caused by C. difficile and seizures.[9]
An increased rate of mortality was observed in people treated with cefiderocol as compared to other antibiotics in a separate clinical trial in critically ill people with multidrug-resistant Gram-negative bacterial infections.[5][9] The higher rate was observed in people treated for hospital-acquired/ventilator-associated pneumonia (i.e., nosocomial pneumonia), bloodstream infections, or sepsis. The cause of death has not been established, but some of the deaths were a result of worsening or complications of infection, or underlying co-morbidities.[5] The safety and efficacy of cefiderocol has not been established for the treatment of these types of infections.[5] Hence, cefiderocol label includes a warning regarding the higher all-cause mortality rate.[5]
In 2021, the first cases of antibiotic resistance were reported,[18] and as of 2022 alarming proportions of up to 50% of resistance in some cohorts have been reported. In September 2022, the German RKI reported victims of the Russian invasion of Ukraine with cefiderocol resistant surgical infections (Klebsiella species and Acinetobacter baumannii) who had been treated in Germany.[19]
Pharmacology
[edit]Its structure is similar to cefepime and ceftazidime, but a chlorocatechol group at the end of the C-3 side chain further enhances its β-lactamase stability and renders it a siderophore.[20]
This means it enters into bacterial cells by binding to iron, which is actively transported into the bacterial cells.[1][21][22][23][24] It was the first siderophore antibiotic to be approved by the U.S. Food and Drug Administration (FDA).[25] It bypasses the bacterial porin channels by using the bacteria's own iron-transport system for being transported in.[26] Once within the periplasmic space, cefiderocol dissociates from the iron and binds to PBPs, inhibiting peptidoglycan cell wall synthesis.[27]
Cefiderocol shows high stability against β-lactamases, with a broad spectrum of activity against all classes of carbapenemases, including serine-carbapenemases (class A such as KPC and class D such as OXA-48) and metallo-β-lactamases (class B such as VIM, NDM, and IMP).[28]
In vitro activity
[edit]Cefiderocol utilises the iron transport system of the bacteria using the “Trojan horse” mechanism. To ensure accurate antibiotic susceptibility testing, cefiderocol requires liquid growth media with low iron levels.[29] According to European Committee on Antimicrobial Susceptibility Testing guidelines, broth microdilution should be performed using iron-depleted Mueller-Hinton broth and specific criteria apply for reading and interpreting the results; specifically, trailing should be disregarded and a growth button of less than 1 mm or light turbidity should be considered as growth.[30]
History
[edit]Cefiderocol received a Qualified Infectious Disease Product designation from the U.S. Food and Drug Administration (FDA) and was granted priority review.[5] In November 2019, the FDA granted approval of cefiderocol to Shionogi & Co. as an antibacterial drug for treatment of adults 18 years of age or older with complicated urinary tract infections, including kidney infections caused by susceptible Gram-negative microorganisms, who have limited or no alternative treatment options.[5][11][1]
The safety and effectiveness of cefiderocol was demonstrated in a study of 448 participants with complicated urinary tract infections.[5][9] Of the participants who were administered cefiderocol, 72.6% had resolution of symptoms and eradication of the bacteria approximately seven days after completing treatment, compared with 54.6% in participants who received an alternative antibiotic.[5] The clinical response rates were similar between the two treatment groups.[5] The trial included participants from Europe, United States and Mexico.[9]
In the clinical trial, participants with complicated urinary tract infections were chosen at random to receive cefiderocol or another antibacterial drug called imipenem/cilastatin.[9] Both treatments were given intravenously for 7–14 days and neither the participants nor the health care professionals knew which drugs were given until after the trial was complete.[9] Participants could not be switched to an oral antibacterial drug to complete the treatment.[9] The benefit of cefiderocol was measured by the proportion of participants who achieved cure or improvement in their symptoms related to complicated urinary tract infections and a negative urine culture test in comparison to imipenem/cilastatin.[9]
In April 2020, cefiderocol was approved for medical use in the European Union.[2]
References
[edit]- ^ a b c d e "Fetroja- cefiderocol sulfate tosylate injection, powder, for solution". DailyMed. 19 November 2019. Retrieved 29 April 2020.
- ^ a b c d "Fetcroja EPAR". European Medicines Agency (EMA). 24 February 2020. Retrieved 29 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Fetcroja Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ Katsube T, Echols R, Arjona Ferreira JC, Krenz HK, Berg JK, Galloway C (May 2017). "Cefiderocol, a Siderophore Cephalosporin for Gram-Negative Bacterial Infections: Pharmacokinetics and Safety in Subjects With Renal Impairment". Journal of Clinical Pharmacology. 57 (5): 584–591. doi:10.1002/jcph.841. PMC 5412848. PMID 27874971.
- ^ a b c d e f g h i j k l m "FDA approves new antibacterial drug to treat complicated urinary tract infections as part of ongoing efforts to address antimicrobial resistance". U.S. Food and Drug Administration (FDA) (Press release). 14 November 2019. Archived from the original on 16 November 2019. Retrieved 15 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Choi JJ, McCarthy MW (February 2018). "Cefiderocol: a novel siderophore cephalosporin". Expert Opinion on Investigational Drugs. 27 (2): 193–197. doi:10.1080/13543784.2018.1426745. PMID 29318906. S2CID 205768562.
- ^ Aoki T, Yoshizawa H, Yamawaki K, Yokoo K, Sato J, Hisakawa S, et al. (July 2018). "Cefiderocol (S-649266), A new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other gram-negative pathogens including multi-drug resistant bacteria: Structure activity relationship". European Journal of Medicinal Chemistry. 155: 847–868. doi:10.1016/j.ejmech.2018.06.014. PMID 29960205. S2CID 49682995.
- ^ Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Ferreira JC, Ariyasu M, et al. (December 2018). "Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial". The Lancet. Infectious Diseases. 18 (12): 1319–1328. doi:10.1016/S1473-3099(18)30554-1. PMID 30509675. S2CID 54552812.
- ^ a b c d e f g h i "Drug Trials Snapshot: Fetroja". U.S. Food and Drug Administration (FDA). 14 November 2019. Retrieved 29 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b Zhanel GG, Golden AR, Zelenitsky S, Wiebe K, Lawrence CK, Adam HJ, et al. (February 2019). "Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli". Drugs. 79 (3): 271–289. doi:10.1007/s40265-019-1055-2. PMID 30712199. S2CID 59541210.
- ^ a b "Drug Approval Package: Fetroja (cefiderocol)". U.S. Food and Drug Administration (FDA). 19 December 2019. Retrieved 29 April 2020.
- ^ "FDA Approves Fetroja (cefiderocol) for the Treatment of Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia". Drugs.com. Retrieved 29 September 2020.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Matsunaga Y, Echols R, Katsube T, Yamano Y, Ariyasu M, Nagata TD (May 2018). "Cefiderocol (S-649266) for Nosocomial Pneumonia Caused by Gram-Negative Pathogens: Study Design of APEKS-NP, a Phase 3 Double-Blind Parallel-Group Randomized Clinical Trial". American Journal of Respiratory and Critical Care Medicine (Abstract) A3290. doi:10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A3290 (inactive 1 November 2024). Retrieved 20 September 2020.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Choby JE, Ozturk T, Satola SW, Jacob JT, Weiss DS (December 2021). "Does cefiderocol heteroresistance explain the discrepancy between the APEKS-NP and CREDIBLE-CR clinical trial results?". The Lancet. Microbe. 2 (12): e648–e649. doi:10.1016/S2666-5247(21)00271-8. PMC 9743357. PMID 35544102.
- ^ Shields RK, Dorazio AJ, Tiseo G, Squires KM, Leonildi A, Giordano C, et al. (October 2024). "Frequency of cefiderocol heteroresistance among patients treated with cefiderocol for carbapenem-resistant Acinetobacter baumannii infections". JAC-antimicrobial Resistance. 6 (5): dlae146. doi:10.1093/jacamr/dlae146. PMC 11382143. PMID 39253335.
- ^ Liu C, Yi J, Lu M, Yang P, Du C, Jiang F, et al. (March 2024). "Dynamic within-host cefiderocol heteroresistance caused by blaSHV-12 amplification in pandrug-resistant and hypervirulent Klebsiella pneumoniae sequence type 11". Drug Resistance Updates. 73: 101038. doi:10.1016/j.drup.2023.101038. PMID 38181587.
- ^ Choby JE, Ozturk T, Satola SW, et al. Widespread cefiderocol heteroresistance in carbapenem- resistant Gram-negative pathogens. Lancet Infect Dis 2021; 21: 597-598
- ^ "Infektionsmedizinische und chirurgische Herausforderungen durch Carbapenem-resistente bakterielle Erreger bei der Versorgung Kriegsverletzter aus der Ukraine" (PDF). Epidemiologisches Bulletin 36/2022. 8 September 2022.
- ^ Karakonstantis S, Rousaki M, Kritsotakis EI (May 2022). "Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance". Antibiotics. 11 (6): 723. doi:10.3390/antibiotics11060723. PMC 9220290. PMID 35740130.
- ^ Sato T, Yamawaki K (November 2019). "Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin". Clinical Infectious Diseases. 69 (Suppl 7): S538–S543. doi:10.1093/cid/ciz826. PMC 6853759. PMID 31724047.
- ^ Matthews-King A (26 October 2018). "Antibiotic 'Trojan horse' could defeat superbugs causing global medical crisis, trial finds". The Independent. Retrieved 26 October 2018.
- ^ Newey S (26 October 2018). "New 'Trojan horse' drug proves effective against antibiotic resistant bacteria". The Telegraph. ISSN 0307-1235. Retrieved 26 October 2018.
- ^ Simpson DH, Scott P (2017). "Antimicrobial Metallodrugs". In Lo KK (ed.). Inorganic and Organometallic Transition Metal Complexes with Biological Molecules and Living Cells. Elsevier. ISBN 978-0-12-803887-1.
- ^ Saisho Y, Katsube T, White S, Fukase H, Shimada J (March 2018). "Pharmacokinetics, Safety, and Tolerability of Cefiderocol, a Novel Siderophore Cephalosporin for Gram-Negative Bacteria, in Healthy Subjects". Antimicrobial Agents and Chemotherapy. 62 (3): 1–12. doi:10.1128/AAC.02163-17. PMC 5826143. PMID 29311072.
- ^ Ito A, Nishikawa T, Matsumoto S, Yoshizawa H, Sato T, Nakamura R, et al. (December 2016). "Siderophore Cephalosporin Cefiderocol Utilizes Ferric Iron Transporter Systems for Antibacterial Activity against Pseudomonas aeruginosa". Antimicrobial Agents and Chemotherapy. 60 (12): 7396–7401. doi:10.1128/AAC.01405-16. PMC 5119021. PMID 27736756.
- ^ Principe L, Lupia T, Andriani L, Campanile F, Carcione D, Corcione S, et al. (April 2022). "Microbiological, Clinical, and PK/PD Features of the New Anti-Gram-Negative Antibiotics: β-Lactam/β-Lactamase Inhibitors in Combination and Cefiderocol-An All-Inclusive Guide for Clinicians". Pharmaceuticals. 15 (4): 463. doi:10.3390/ph15040463. PMC 9028825. PMID 35455461.
- ^ El-Lababidi RM, Rizk JG (December 2020). "Cefiderocol: A Siderophore Cephalosporin". The Annals of Pharmacotherapy. 54 (12): 1215–1231. doi:10.1177/1060028020929988. PMID 32522005. S2CID 219586396.
- ^ Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF (August 2019). "Reproducibility of broth microdilution MICs for the novel siderophore cephalosporin, cefiderocol, determined using iron-depleted cation-adjusted Mueller-Hinton broth". Diagnostic Microbiology and Infectious Disease. 94 (4): 321–325. doi:10.1016/j.diagmicrobio.2019.03.003. PMID 31029489.
- ^ The European Committee on Antimicrobial Susceptibility Testing. EUCAST Guidance document on broth microdilution testing of cefiderocol. 2024. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Guidance_documents/Cefiderocol_MIC_testing_EUCAST_guidance_document_January_2024.pdf
Further reading
[edit]- Committee for Medicinal Products for Human Use (27 February 2020). "Fetcroja: EPAR - Public assessment report" (PDF). European Medicines Agency (EMA). EMA/136096/2020.
External links
[edit]- "Cefiderocol". Drug Information Portal. U.S. National Library of Medicine.
- "Cefiderocol sulfate tosylate". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02321800 for "A Study of Efficacy and Safety of Intravenous Cefiderocol (S-649266) Versus Imipenem/Cilastatin in Complicated Urinary Tract Infections (APEKS-cUTI)" at ClinicalTrials.gov
