Carbon-based life

Carbon is a primary component of all known life on Earth, and represents approximately 45–50% of all dry biomass.[1] Carbon compounds occur naturally in great abundance on Earth. Complex biological molecules consist of carbon atoms bonded with other elements, especially oxygen and hydrogen and frequently also nitrogen, phosphorus, and sulfur (collectively known as CHNOPS).[2][3]
Because it is lightweight and relatively small in size, carbon molecules are easy for enzymes to manipulate. Carbonic anhydrase is part of this process. Carbon has an atomic number of 6 on the periodic table. The carbon cycle is a biogeochemical cycle that is important in maintaining life on Earth over a long time span. The cycle includes carbon sequestration and carbon sinks.[4][5] Plate tectonics are needed for life over a long time span, and carbon-based life is important in the plate tectonics process.[6] Iron- and sulfur-based Anoxygenic photosynthesis life forms that lived from 3.80 to 3.85 billion years ago on Earth produced an abundance of black shale deposits. These shale deposits increase heat flow and crust buoyancy, especially on the sea floor, helping to increase plate tectonics. Talc is another organic mineral that helps drive plate tectonics.[7][8] Inorganic processes also help drive plate tectonics.[9] Carbon-based photosynthesis life caused a rise in oxygen on Earth. This increase of oxygen helped plate tectonics form the first continents.[10] It is frequently assumed in astrobiology that if life exists elsewhere in the Universe, it will also be carbon-based.[11][12] Critics, like Carl Sagan in 1973, refer to this assumption as carbon chauvinism.[13]
Characteristics
[edit]Carbon is capable of forming a vast number of compounds, more than any other element, with almost ten million compounds described to date,[14] and yet that is but a fraction of the number of compounds that are theoretically possible under standard conditions. The enormous diversity of carbon compounds, known as organic compounds, has led to a distinction between them and the inorganic compounds that do not contain carbon. The branch of chemistry that studies organic compounds is known as organic chemistry.[15]
Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass, after hydrogen, helium, and oxygen. Carbon's widespread abundance, its ability to form stable bonds with numerous other elements, and its unusual ability to form polymers at the temperatures commonly encountered on Earth enables it to serve as a common element of all known living organisms. In a 2018 study, carbon was found to compose approximately 550 billion tons of all life on Earth.[16][17] It is the second most abundant element in the human body by mass (about 18.5%) after oxygen.[18]
The most important characteristics of carbon as a basis for the chemistry of cellular life are that each carbon atom is capable of forming up to four valence bonds with other atoms simultaneously, and that the energy required to make or break a bond with a carbon atom is at an appropriate level for building large and complex molecules which may be both stable and reactive.[19] Carbon atoms bond readily to other carbon atoms; this allows the building of arbitrarily long macromolecules and polymers in a process known as catenation.[20][21][22] "What we normally think of as 'life' is based on chains of carbon atoms, with a few other atoms, such as nitrogen or phosphorus", per Stephen Hawking in a 2008 lecture, "carbon [...] has the richest chemistry."[23]
Norman Horowitz was the head of the Jet Propulsion Laboratory's bioscience section for the first U.S. mission, Viking Lander of 1976, to successfully land an unmanned probe on the surface of Mars. He considered that the great versatility of the carbon atom makes it the element most likely to provide solutions, even exotic solutions, to the problems of survival on other planets. However, the results of this mission indicated that Mars was presently extremely hostile to carbon-based life. He also considered that, in general, there was only a remote possibility that non-carbon life forms would be able to evolve with genetic information systems capable of self-replication and adaptation.[24]
Key molecules
[edit]The most notable classes of biological macromolecules used in the fundamental processes of living organisms include:[25]
- Proteins, which are the building blocks from which the structures of living organisms are constructed (this includes almost all enzymes, which catalyse organic chemical reactions).[2]
- Amino acid, make up proteins, included the use in genetic code of life.[2]
- Nucleic acids, which carry genetic information.[2]
- Ribonucleic acid (RNA), production of proteins.[26]
- Deoxyribonucleic acid (DNA), nucleic acid in genetic form.[27]
- Peptide, building block of proteins.[2]
- Lipids, which also store energy, but in a more concentrated form, and which may be stored for extended periods in the bodies of animals.[2]
- Phospholipid used in cell membrane.[2]
- Carbohydrates, which store energy in a form that can be used by living cells.[2]
- Lectin, for binding proteins.[28]
- Monosaccharide, simple sugars, including glucose and fructose.[2]
- Disaccharides, sugar soluble in water, including lactose, maltose, and sucrose.[2]
- Starch, made of amylose and amylopectin, plants energy storage.[2]
- Glycogen, energy in animals.[2]
- Cellulose, a biopolymer, found in the cell walls of plants.[2]
- Fatty acid, two types, saturated fat and unsaturated fat (oil), are stored energy.[2]
- Essential fatty acid, needed but not synthesized by the human body.[2]
- Steroid, hormone, and used in cell membrane.[2]
- Neurotransmitter, are signaling molecules.[29]
- Cholesterol, used in the brain and spinal cord of animals.[2]
- Wax, found in beeswax and lanolin. Plant wax used for protection.[2]
Water
[edit]
Liquid water is essential for carbon-based life. Chemical bonding of carbon molecules requires liquid water.[30] Water has the chemical property to make compound-solvent pairing.[31] Water provides the reversible hydration of carbon dioxide. Hydration of carbon dioxide is needed in carbon-based life. All life on Earth uses the same biochemistry of carbon. Water is important in life's carbonic anhydrase the interaction of between carbon dioxide and water. Carbonic anhydrase needs a family of carbon base enzymes for the hydration of carbon dioxide and acid–base homeostasis, that regulates PH levels in life. [32][33] In plant life, liquid water is needed for photosynthesis, the biological process plants use to convert light energy and carbon dioxide into chemical energy.[34] Water makes up 55% to 60% of the human body by weight.[35]
Other candidates
[edit]A few other elements have been proposed as candidates for supporting biological systems and processes as fundamentally as carbon does, for example, processes such as metabolism. The most frequently suggested alternative is silicon.[36] Silicon, atomic number of 14, more than twice the size of carbon, shares a group in the periodic table with carbon, can also form four valence bonds, and also bonds to itself readily, though generally in the form of crystal lattices rather than long chains. Despite these similarities, silicon is considerably more electropositive than carbon, and silicon compounds do not readily recombine into different permutations in a manner that would plausibly support lifelike processes. Silicon is abundant on Earth, but as it is more electropositive and in a water based environment it forms Si–O bonds rather than Si–Si bonds.[37] Boron does not react with acids and does not form chains naturally. Thus boron is not a candidate for life.[38] Arsenic is toxic to life, and its possible candidacy has been rejected.[39][40] In the past (1960s-1970s) other candidates for life were plausible, but with time and more research, only carbon has the complexity and stability to make large molecules and polymers essential for life.[41][42][43]
Fiction
[edit]Speculations about the chemical structure and properties of hypothetical non-carbon-based life have been a recurring theme in science fiction. Silicon is often used as a substitute for carbon in fictional lifeforms because of its chemical similarities. In cinematic and literary science fiction, when man-made machines cross from non-living to living, this new form is often presented as an example of non-carbon-based life. Since the advent of the microprocessor in the late 1960s, such machines are often classed as "silicon-based life". Other examples of fictional "silicon-based life" can be seen in the 1967 episode "The Devil in the Dark" from Star Trek: The Original Series, in which a living rock creature's biochemistry is based on silicon.[44] In the 1994 The X-Files episode "Firewalker", in which a silicon-based organism is discovered in a volcano.[45][46]
In the 1984 film adaptation of Arthur C. Clarke's 1982 novel 2010: Odyssey Two, a character argues, "Whether we are based on carbon or on silicon makes no fundamental difference; we should each be treated with appropriate respect."[47]
In JoJolion, the eighth part of the larger JoJo's Bizarre Adventure series, a mysterious race of silicon-based lifeforms "Rock Humans" serve as the primary antagonists.[48]
Gallery
[edit]-
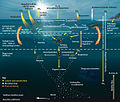
Correlation between the carbon cycle and formation of organic compounds.
-
Plant cell wall (cellulose) and chloroplasts conduct photosynthesis in plant cells and other eukaryotic organisms.
-
The arrangement of cellulose and other polysaccharides in a plant cell wall
-
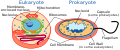
Cell types: eukaryotic cell (left) and prokaryotic cell (right)
-
Fast carbon cycle showing the movement of carbon between land, atmosphere
-
Carbon stored in ecosystems
-
Where carbon goes when water flows
-
Simplified diagram of the global carbon cycle
-
Carbon cycle diagram
-
Triple bond of Carbon in Benzene
-
Model of diisobutylaluminium hydride, showing aluminium as pink, bonded to carbon in black, and hydrogen as white in Organoaluminium chemistry
-
Fatty acids made of long chains of carbon
See also
[edit]- Carbon source (biology)
- Cell biology
- CHONPS, a mnemonic acronym for the order of the most common elements in living organisms: carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur
- Habitable zone for complex life
References
[edit]- ^ "Knowledge reference for national forest assessments - modeling for estimation and monitoring". www.fao.org. Archived from the original on January 13, 2020. Retrieved Feb 20, 2019.
- ^ a b c d e f g h i j k l m n o p q r Molnar, Charles; Gair, Jane (May 14, 2015). "2.3 Biological Molecules". Introduction to the Chemistry of Life – via opentextbc.ca.
- ^ Education (2010). "CHNOPS: The Six Most Abundant Elements of Life". Pearson Education. Pearson BioCoach. Archived from the original on 27 July 2017. Retrieved 2010-12-10.
Most biological molecules are made from covalent combinations of six important elements, whose chemical symbols are CHNOPS. ... Although more than 25 types of elements can be found in biomolecules, six elements are most common. These are called the CHNOPS elements; the letters stand for the chemical abbreviations of carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur.
- ^ Riebeek, Holli (16 June 2011). "The Carbon Cycle". Earth Observatory. NASA. Archived from the original on 5 March 2016. Retrieved 5 April 2018.
- ^ Archer, David (2010). The global carbon cycle. Princeton: Princeton University Press. ISBN 9781400837076.
- ^ "How plate tectonics have maintained Earth's 'Goldilocks' climate". The University of Sydney.
- ^ "Talc Processing". www.soapstonetalc.com.
- ^ Sidder, Aaron (August 23, 2023). "Talc May Make Mexico's Subduction Zone More Slippery". Eos.
- ^ "Geology, Age and Origin of Supracrustal Rocks at Akilia, West Greenland".
- ^ Bressan, David. "Rise Of Oxygen On Early Earth Linked To The Formation Of First Continents". Forbes.
- ^ "Astrobiology". Biology Cabinet. September 26, 2006. Retrieved 2011-01-17.
- ^ "Polycyclic Aromatic Hydrocarbons: An Interview With Dr. Farid Salama". Astrobiology magazine. 2000. Archived from the original on 2008-06-20. Retrieved 2008-10-20.
- ^ Darling, David. "Carbon-based life". Encyclopedia of Life. Retrieved 14 September 2007.
- ^ "There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes." Chemistry Operations (December 15, 2003). "Carbon". Los Alamos National Laboratory. Archived from the original on 2008-09-13. Retrieved 2008-10-09.
- ^ Clayden, J.; Greeves, N. and Warren, S. (2012) Organic Chemistry. Oxford University Press. pp. 1–15. ISBN 0-19-927029-5.
- ^ Bar-On, Yinon M.; Phillips, Rob; Milo, Ron (May 21, 2018). "The biomass distribution on Earth". Proceedings of the National Academy of Sciences. 115 (25): 6506–6511. Bibcode:2018PNAS..115.6506B. doi:10.1073/pnas.1711842115. PMC 6016768. PMID 29784790.
- ^ Carrington, Damian (May 21, 2018). "Humans just 0.01% of all life but have destroyed 83% of wild mammals – study". The Guardian. Retrieved Feb 20, 2019 – via www.theguardian.com.
- ^ Reece, Jane B. (31 October 2013). Campbell Biology (10 ed.). Pearson. ISBN 9780321775658.
- ^ "Carbon and hydrocarbons (article)". Khan Academy.
- ^ Oxford English Dictionary, 1st edition (1889) [http://www.oed.com/view/Entry/30197 s.v. 'chain', definition 4g
- ^ "27.8: Polymers and Polymerization Reactions". Chemistry LibreTexts. January 18, 2015.
- ^ "Polymers". www2.chemistry.msu.edu.
- ^ Stephen Hawking (1 October 2008). "Life in the Universe, 50th anniversary celebration of NASA". NASA. Retrieved 28 August 2015.
- ^ Horowitz, N.H. (1986). Utopia and Back and the search for life in the solar system. New York: W.H. Freeman and Company. ISBN 0-7167-1766-2
- ^ Molnar, Charles; Gair, Jane (2015-05-14). "2.3 Biological Molecules". Introduction to the Chemistry of Life.
- ^ "RNA: The Versatile Molecule". University of Utah. 2015.
- ^ "deoxyribonucleic acid". Merriam-Webster.com Dictionary. Merriam-Webster.
- ^ URS Rutishauser; Leo Sachs (May 1, 1975). "Cell-to-Cell Binding Induced by Different Lectins". Journal of Cell Biology. 65 (2): 247–257. doi:10.1083/jcb.65.2.247. PMC 2109424. PMID 805150.
- ^ Smelser, Neil J.; Baltes, Paul B. (2001). International encyclopedia of the social & behavioral sciences (1st ed.). Amsterdam New York: Elsevier. ISBN 978-0-08-043076-8.
- ^ "Eight ingredients for life in space". www.nhm.ac.uk.
- ^ Westall, Frances; Brack, André (March 1, 2018). "The Importance of Water for Life". Space Science Reviews. 214 (2): 50. Bibcode:2018SSRv..214...50W. doi:10.1007/s11214-018-0476-7. S2CID 255068746 – via NASA ADS.
- ^ "Reactome | Reversible hydration of carbon dioxide". reactome.org.
- ^ "Carbon-Based Life - an overview | ScienceDirect Topics". www.sciencedirect.com.
- ^ "Photosynthesis". Lexico UK English Dictionary. Oxford University Press. Archived from the original on 2022-08-11. Retrieved 2023-07-15.
- ^ "The Water in You: Water and the Human Body | U.S. Geological Survey". www.usgs.gov.
- ^ Pace, NR (2001). "The universal nature of biochemistry". Proceedings of the National Academy of Sciences of the United States of America. 98 (3): 805–8. Bibcode:2001PNAS...98..805P. doi:10.1073/pnas.98.3.805. PMC 33372. PMID 11158550.
- ^ Petkowski, Janusz Jurand; Bains, William; Seager, Sara (June 10, 2020). "On the Potential of Silicon as a Building Block for Life". Life. 10 (6): 84. Bibcode:2020Life...10...84P. doi:10.3390/life10060084. PMC 7345352. PMID 32532048.
- ^ "The Boron Family & Its Physical and Chemical Properties | PDF | Carbon | Silicon". Scribd.
- ^ Check Hayden, Erika (January 20, 2012). "Study challenges existence of arsenic-based life". Nature. doi:10.1038/nature.2012.9861. S2CID 211729481 – via www.nature.com.
- ^ Sheridan, Kerry. "Scientists say NASA's 'new arsenic form of life' was untrue". phys.org.
- ^ Aono, Masashi; Kitadai, Norio; Oono, Yoshi (February 12, 2015). "A Principled Approach to the Origin Problem". Origins of Life and Evolution of the Biosphere. 45 (3): 327–338. Bibcode:2015OLEB...45..327A. doi:10.1007/s11084-015-9444-3. PMC 4510921. PMID 26177711.
- ^ "The Unique Carbon Atom, National Oceanic and Atmospheric Administration, noaa.gov" (PDF).
- ^ "Biology, The Chemistry of Life, The Chemical Foundation of Life, Carbon". OERTX.
- ^ "Star Trek | The Science of Silicon-Based Life". The Companion. March 30, 2022.
- ^ Lowry, Brian (1995). The Truth is Out There: The Official Guide to the X-Files. Harper Prism. ISBN 0-06-105330-9.
- ^ Edwards, Ted (1996). X-Files Confidential. Little, Brown and Company. ISBN 0-316-21808-1.
- ^ "2010: Quotes". IMDb. Archived from the original on 12 January 2017. Retrieved 26 July 2017.
- ^ "Rock Organism". JoJo's Bizarre Encyclopedia - JoJo Wiki. 23 November 2023.