Capillin
Appearance
 | |
| Names | |
|---|---|
| Preferred IUPAC name
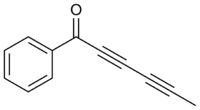
1-Phenylhexa-2,4-diyn-1-one | |
| Other names
Capillin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H8O | |
| Molar mass | 168.195 g·mol−1 |
| Melting point | 82–83 °C |
| 0.0177 mg/mL | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Capillin is a naturally occurring organic compound with the chemical formula C
12H
8O. The structure contains acetophenone and a polyyne (pentadiynyl) portion, conjugated together as an ynone.
Chemical taxonomy
[edit]Capillin is found in the essential oil of a number of Artemisia species, including Artemisia monosperma and Artemisia dracunculus (tarragon).[1] The substance was initially isolated from Artemisia capillaris in 1956.[2]
Applications
[edit]Capillin is a biologically active substance. It has strong antifungal activity, and it is possibly antitumoral. Capillin exhibits cytotoxic activity and could cause apoptosis of certain human tumor cells.[3]
References
[edit]- ^ Wishart, David S.; Guo, An Chi; Oler, Eponine; Wang, Fel; Anjum, Afia; Peters, Harrison; Dizon, Raynard; Sayeeda, Zinat; Tian, Siyang; Lee, Brian L.; Berjanskii, Mark; Mah, Robert; Yamamoto, Mai; Jovel Castillo, Juan; Torres Calzada, Claudia; Hiebert Giesbrecht, Mickel; Lui, Vicki W.; Varshavi, Dorna; Varshavi, Dorsa; Allen, Dana; Arndt, David; Khetarpal, Nitya; Sivakumaran, Aadhavya; Harford, Karxena; Sanford, Selena; Yee, Kristen; Cao, Xuan; Budinsky, Zachary; Liigand, Jaanus; Zhang, Lun; Zheng, Jiamin; Mandal, Rupasri; Karu, Naama; Dambrova, Maija; Schiöth, Helgi B.; Gautam, Vasuk. "Showing metabocard for Capillin (HMDB32867)". Human Metabolome Database, HMDB. 5.0.
- ^ Nash, B. W.; Thomas, D. A.; Warburton, W. K.; Williams, Thelma D. (1965). "535. The preparation of capillin and some related compounds, and of some substituted pent-4-en-2-yn-1-ones". J. Chem. Soc.: 2983–2988. doi:10.1039/JR9650002983.
- ^ Whelan LC, Ryan MF (2004). "Effects of the polyacetylene capillin on human tumour cell lines". Anticancer Research. 24 (4): 2281–6. PMID 15330173.
