Chloropyramine
This article includes a list of general references, but it lacks sufficient corresponding inline citations. (June 2013) |
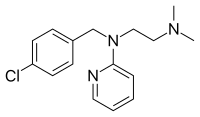 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular, topical |
| Drug class | First-generation antihistamine |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100%[1] |
| Metabolism | Extensive hepatic |
| Onset of action | 15–30 min (oral)[2] |
| Elimination half-life | ~14 hours[2] |
| Excretion | Kidney[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.383 |
| Chemical and physical data | |
| Formula | C16H20ClN3 |
| Molar mass | 289.81 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Chloropyramine is a first-generation antihistamine drug approved in several Eastern European countries such as Russia for the treatment of allergic conjunctivitis, allergic rhinitis, bronchial asthma, and other atopic (allergic) conditions. Related indications for clinical use include angioedema, allergic reactions to insect bites, food and drug allergies, and anaphylactic shock.[citation needed]
Chloropyramine is known as a competitive reversible H1 receptor antagonist (also known as an H1 inverse agonist), meaning that it exerts its pharmacological action by competing with histamine for the H1 subtype histamine receptor. By blocking the effects of histamine, the drug inhibits the vasodilation, increased vascular permeability, and tissue edema associated with histamine release in the tissue. The H1 antagonistic properties of chloropyramine can be used by researchers for the purposes of blocking the effects of histamine on cells and tissues. In addition, chloropyramine has some anticholinergic properties.[1]
Chloropyramine's anticholinergic properties and the fact that it can pass through the blood–brain barrier are linked to its clinical side effects such as drowsiness, weakness, vertigo, fatigue, dry mouth, constipation, and rarely — visual disturbances and increase of intraocular pressure.[citation needed]
Contraindications
[edit]Contraindications for parenteral or oral administration include benign prostatic hyperplasia, peptic ulcer, pyloric and duodenal stenosis, uncontrolled glaucoma, pregnancy and breast-feeding. It is not intended for the management of acute bronchospasm.[1] It should be used with caution in patients with hyperthyroidism, cardiovascular diseases, and asthma.[citation needed]
Adverse effects
[edit]In children, it can induce agitation, and in many adult patients, dizziness may be observed. Because of the pronounced sedative effect, the preparation should be prescribed cautiously in drivers and people working with machines.[citation needed]
A large study on people 65 years old or older linked the development of Alzheimer's disease and other forms of dementia to the "higher cumulative" use of first-generation antihistamines, due to their anticholinergic properties.[3]
Interactions
[edit]Chloropyramine should not be used internally with MAO inhibitors. Because of its anticholinergic activity, concurrent administration with cholinomimetics is not advisable. Chloropyramine should also not be used internally with alcohol, sedatives, and hypnotics because of the potentiation of the effects. General anesthetics, opioids, and other depressants potentiate the sedative effect of chloropyramine.[citation needed]
Dosage
[edit]In cases of severe allergic reactions, chloropyramine can be injected intramuscularly or intravenously. Oral administration: In adults, 25 mg can be taken 3 to 4 times daily (up to 150 mg); in children over 5 years old, 25 mg can be taken 2 to 3 times daily. For external application, the skin or the eye conjunctiva can be treated up to several times a day by applying a thin layer of cream or ointment containing 1% chloropyramine hydrochloride.[citation needed]
Trade names
[edit]- Allergopress, Chimpharm AD (KZ)
- Allergosan, Sopharma AD (BG, GE, LV)
- Suprastin, Egis Pharmaceuticals PLC (GE, HU, KZ, LT, LV, UA, RU)
- Supralgon, Biopharm JSC (GE)
- Supranorm-Tsiteli A, Rompharm Co. (GE)
- Synopen, Pliva d.o.o. (BA, HR, RS,MK)
Synthesis
[edit]
The preparation begins with the condensation of 4-chlorobenzaldehyde with 1,1-dimethyethylenediamine. The resulting Schiff base is reduced. The resulting amine is then further reacts with 2-bromopyridine in the presence of sodamide.


See also
[edit]- Mepyramine (4-methoxy analog)
- Methapyrilene
- Chlorothen
References
[edit]- ^ a b c "Chloropyramine Tablets for Oral Use. Prescribing Information". State Register of Medicines (in Russian). Ozon OOO. Retrieved 5 January 2016.
- ^ a b c "Хлоропирамин (Chloropyraminum)" (in Russian).
- ^ Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. (March 2015). "Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study". JAMA Internal Medicine. 175 (3): 401–407. doi:10.1001/jamainternmed.2014.7663. PMC 4358759. PMID 25621434.
- ^ Vaughan JR, Anderson GW (March 1949). "Antihistamine agents; halogenated N,N-dimethyl-N-benzyl-N-(2-pyridyl)-ethylenediamines". The Journal of Organic Chemistry. 14 (2): 228–34. doi:10.1021/jo01154a006. PMID 18117722.
- ^ US granted 2569314, Kenneth HL, "N, n-dimethyl-n'-(4-halobenzyl)-n'-(2-pyridyl) ethylenediamines and preparation of the same", issued 1951, assigned to American Cyanamide
- ^ US granted 2607778, Cates EM, Phillips RF, "Process of preparing n, n-dimethyln'-(p-chlorobenzyl)-n'-(2-pyridyl)-ethylenediamine", issued 1952, assigned to Merck & Co.
- ^ CH granted 264754, "Verfahren zur Herstellung eines Äthylendiaminderivates.", issued 1950, assigned to J. R. Geigy AG
- ^ CH granted 266234, "Verfahren zur Herstellung eines Äthylendiaminderivates.", issued 1950, assigned to J. R. Geigy AG
- ^ CH granted 266235, "Verfahren zur Herstellung eines Äthylendiaminderivates.", issued 1950, assigned to J. R. Geigy AG
- ^ GB granted 651596, "Manufacture of n-(2-pyridyl)-n-(p-chloro-benzyl)-n:n-dimethyl-ethylenediamine", issued 1951, assigned to J. R. Geigy AG
