Antiandrogen
| Antiandrogen | |
|---|---|
| Drug class | |
 Bicalutamide, a nonsteroidal antiandrogen and the most widely used androgen receptor antagonist in the treatment of prostate cancer. | |
| Class identifiers | |
| Synonyms | Androgen antagonists; Androgen blockers; Testosterone blockers |
| Use | • Men and boys: Prostate cancer; Benign prostatic hyperplasia; Scalp hair loss; Paraphilias; Hypersexuality; Sex offenders; Precocious puberty; Priapism • Women and girls: Acne; Seborrhea; Hidradenitis suppurativa; Hirsutism; Scalp hair loss; Hyperandrogenism; Transgender hormone therapy |
| ATC code | L02BB |
| Biological target | Androgen receptor; Progesterone receptor; Estrogen receptor; GnRH receptor; 5α-Reductase; CYP17A1 (17α-hydroxylase/ |
| Chemical class | Steroidal; Nonsteroidal; Peptide |
| External links | |
| MeSH | D000726 |
| Legal status | |
| In Wikidata | |
Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production.[1][2] They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.[3]
Antiandrogens are used to treat an assortment of androgen-dependent conditions.[4] In men, antiandrogens are used in the treatment of prostate cancer, enlarged prostate, scalp hair loss, overly high sex drive, unusual and problematic sexual urges, and early puberty.[4][5] In women, antiandrogens are used to treat acne, seborrhea, excessive hair growth, scalp hair loss, and high androgen levels, such as those that occur in polycystic ovary syndrome (PCOS).[4] Antiandrogens are also used as a component of feminizing hormone therapy for transgender women and as puberty blockers in transgender girls.[4]
Side effects of antiandrogens depend on the type of antiandrogen and the specific antiandrogen in question. In any case, common side effects of antiandrogens in men include breast tenderness, breast enlargement, feminization, hot flashes, sexual dysfunction, infertility, and osteoporosis. In women, antiandrogens are much better tolerated, and antiandrogens that work only by directly blocking androgens are associated with minimal side effects. However, because estrogens are made from androgens in the body, antiandrogens that suppress androgen production can cause low estrogen levels and associated symptoms like hot flashes, menstrual irregularities, and osteoporosis in premenopausal women.
There are a few different major types of antiandrogens.[6] These include AR antagonists, androgen synthesis inhibitors, and antigonadotropins.[6] AR antagonists work by directly blocking the effects of androgens, while androgen synthesis inhibitors and antigonadotropins work by lowering androgen levels.[6] AR antagonists can be further divided into steroidal antiandrogens and nonsteroidal antiandrogens; androgen synthesis inhibitors can be further divided mostly into CYP17A1 inhibitors and 5α-reductase inhibitors; and antigonadotropins can be further divided into gonadotropin-releasing hormone modulators (GnRH modulators), progestogens, and estrogens.[6][7][8]
Medical uses
[edit]Antiandrogens are used in the treatment of an assortment of androgen-dependent conditions in both males and females.[4][9] They are used to treat men with prostate cancer, benign prostatic hyperplasia, pattern hair loss, hypersexuality, paraphilias, and priapism, as well as boys with precocious puberty.[9][10][11] In women and girls, antiandrogens are used to treat acne, seborrhea, hidradenitis suppurativa, hirsutism, and hyperandrogenism.[9][12][13] Antiandrogens are also used in transgender women as a component of feminizing hormone therapy and as puberty blockers in transgender girls.[14][15]
Men and boys
[edit]Prostate cancer
[edit]Androgens like testosterone and particularly DHT are importantly involved in the development and progression of prostate cancer.[16] They act as growth factors in the prostate gland, stimulating cell division and tissue growth.[16] In accordance, therapeutic modalities that reduce androgen signaling in the prostate gland, referred to collectively as androgen deprivation therapy, are able to significantly slow the course of prostate cancer and extend life in men with the disease.[16] Although antiandrogens are effective in slowing the progression of prostate cancer, they are not generally curative, and with time, the disease adapts and androgen deprivation therapy eventually becomes ineffective.[17] When this occurs, other treatment approaches, such as chemotherapy, may be considered.[17]
The most common methods of androgen deprivation therapy currently employed to treat prostate cancer are castration (with a GnRH modulator or orchiectomy), nonsteroidal antiandrogens, and the androgen synthesis inhibitor abiraterone acetate.[16] Castration may be used alone or in combination with one of the other two treatments.[16][18] When castration is combined with a nonsteroidal antiandrogen like bicalutamide, this strategy is referred to as combined androgen blockade (also known as complete or maximal androgen blockade).[16][19] Enzalutamide, apalutamide, and abiraterone acetate are specifically approved for use in combination with castration to treat castration-resistant prostate cancer.[16][20] Monotherapy with the nonsteroidal antiandrogen bicalutamide is also used in the treatment of prostate cancer as an alternative to castration with comparable effectiveness but with a different and potentially advantageous side effect profile.[16][21][22]
High-dose estrogen was the first functional antiandrogen used to treat prostate cancer. It was widely used, but has largely been abandoned for this indication in favor of newer agents with improved safety profiles and fewer feminizing side effects.[23] Cyproterone acetate was developed subsequently to high-dose estrogen and is the only steroidal antiandrogen that has been widely used in the treatment of prostate cancer,[24] but it has largely been replaced by nonsteroidal antiandrogens, which are newer and have greater effectiveness, tolerability, and safety.[25][26] Bicalutamide, as well as enzalutamide, have largely replaced the earlier nonsteroidal antiandrogens flutamide and nilutamide, which are now little used.[19][27][28][29][30] The earlier androgen synthesis inhibitors aminoglutethimide and ketoconazole have only limitedly been used in the treatment of prostate cancer due to toxicity concerns and have been replaced by abiraterone acetate.[31]
In addition to active treatment of prostate cancer, antiandrogens are effective as prophylaxis (preventatives) in reducing the risk of ever developing prostate cancer.[32] Antiandrogens have only limitedly been assessed for this purpose, but the 5α-reductase inhibitors finasteride and dutasteride and the steroidal AR antagonist spironolactone have been associated with significantly reduced risk of prostate cancer.[32][33] In addition, it is notable that prostate cancer is extremely rare in transgender women who have been on feminizing hormone therapy for an extended period of time.[34][35][36]
Enlarged prostate
[edit]The 5α-reductase inhibitors finasteride and dutasteride are used to treat benign prostatic hyperplasia, a condition in which the prostate becomes enlarged and this results in urinary obstruction and discomfort.[37] They are effective because androgens act as growth factors in the prostate gland.[37] The antiandrogens chlormadinone acetate and oxendolone and the functional antiandrogens allylestrenol and gestonorone caproate are also approved in some countries for the treatment of benign prostatic hyperplasia.[38][39]
Scalp hair loss
[edit]5α-Reductase inhibitors like finasteride, dutasteride, and alfatradiol and the topical nonsteroidal AR antagonist topilutamide (fluridil) are approved for the treatment of pattern hair loss, also known as scalp hair loss or baldness.[40] This condition is generally caused by androgens, so antiandrogens can slow or halt its progression.[41] Systemic antiandrogens besides 5α-reductase inhibitors are not generally used to treat scalp hair loss in males due to risks like feminization (e.g., gynecomastia) and sexual dysfunction.[42][43][44][45][46][47][48] However, they have been assessed and reported to be effective for this indication.[42][43][49]
Acne
[edit]Systemic antiandrogens are generally not used to treat acne in males due to their high risk of feminization (e.g., gynecomastia) and sexual dysfunction.[50][51] However, they have been studied for acne in males and found to be effective.[52][44][45][53] Clascoterone, a topical antiandrogen, is effective for acne in males and has been approved by the FDA in August 2020.[54][55][56][57]
Paraphilia
[edit]Androgens increase sex drive,[58] and for this reason, antiandrogens are able to reduce sex drive in men.[59][60] In accordance, antiandrogens are used in the treatment of conditions such as hypersexuality (excessively high sex drive) and paraphilias (atypical and sometimes societally unacceptable sexual interests) like pedophilia (sexual attraction to children).[59][60] They have been used to decrease sex drive in sex offenders so as to reduce the likelihood of recidivism (repeat offenses).[61] Antiandrogens used for these indications include cyproterone acetate, medroxyprogesterone acetate, and GnRH modulators.[62][63]
Early puberty
[edit]Antiandrogens are used to treat precocious puberty in boys.[64][65][66][67] They work by opposing the effects of androgens and delaying the development of secondary sexual characteristics and onset of changes in sex drive and function until a more appropriate age.[64][65] Antiandrogens that have been used for this purpose include cyproterone acetate, medroxyprogesterone acetate, GnRH modulators, spironolactone, bicalutamide, and ketoconazole.[64][67][68][69][70][71] Spironolactone and bicalutamide require combination with an aromatase inhibitor to prevent the effects of unopposed estrogens, while the others can be used alone.[64][70][71]
Long-lasting erections
[edit]Antiandrogens are effective in the treatment of recurrent priapism (potentially painful penile erections that last more than four hours).[72][73][74][75][76]
Women and girls
[edit]Skin and hair conditions
[edit]Antiandrogens are used in the treatment of androgen-dependent skin and hair conditions including acne, seborrhea, hidradenitis suppurativa, hirsutism, and pattern hair loss in women.[12] All of these conditions are dependent on androgens, and for this reason, antiandrogens are effective in treating them.[12] The most commonly used antiandrogens for these indications are cyproterone acetate and spironolactone.[77] Flutamide has also been studied extensively for such uses, but has fallen out of favor due to its association with hepatotoxicity.[78] Bicalutamide, which has a relatively minimal risk of hepatotoxicity, has been evaluated for the treatment of hirsutism and found effective similarly to flutamide and may be used instead of it.[79][80] In addition to AR antagonists, oral contraceptives containing ethinylestradiol are effective in treating these conditions, and may be combined with AR antagonists.[81][82]
High androgen levels
[edit]Hyperandrogenism is a condition in women in which androgen levels are excessively and abnormally high.[13] It is commonly seen in women with PCOS, and also occurs in women with intersex conditions like congenital adrenal hyperplasia.[13] Hyperandrogenism is associated with virilization – that is, the development of masculine secondary sexual characteristics like male-pattern facial and body hair growth (or hirsutism), voice deepening, increased muscle mass and strength, and broadening of the shoulders, among others.[13] Androgen-dependent skin and hair conditions like acne and pattern hair loss may also occur in hyperandrogenism, and menstrual disturbances, like amenorrhea, are commonly seen.[13] Although antiandrogens do not treat the underlying cause of hyperandrogenism (e.g., PCOS), they are able to prevent and reverse its manifestation and effects.[13] As with androgen-dependent skin and hair conditions, the most commonly used antiandrogens in the treatment of hyperandrogenism in women are cyproterone acetate and spironolactone.[13] Other antiandrogens, like bicalutamide, may be used alternatively.[13]
Gender-affirming hormone therapy
[edit]Antiandrogens are used to prevent or reverse masculinization and to facilitate feminization in transgender women and some nonbinary individuals who are undergoing hormone therapy and who have not undergone sex reassignment surgery or orchiectomy.[14] Besides estrogens, the main antiandrogens that have been used for this purpose are cyproterone acetate, spironolactone, and GnRH modulators.[14] Nonsteroidal antiandrogens like bicalutamide are also used for this indication.[83][14] In addition to use in transgender women, antiandrogens, mainly GnRH modulators, are used as puberty blockers to prevent the onset of puberty in transgender girls until they are older and ready to begin hormone therapy.[15]
Available forms
[edit]There are several different types of antiandrogens, including the following:[6]
- Androgen receptor antagonists: Drugs that bind directly to and block the AR.[84][85] These drugs include the steroidal antiandrogens cyproterone acetate, megestrol acetate, chlormadinone acetate, spironolactone, oxendolone, and osaterone acetate (veterinary) and the nonsteroidal antiandrogens flutamide, bicalutamide, nilutamide, topilutamide, enzalutamide, apalutamideand Finasteride.[84][85][7][8] Aside from cyproterone acetate and chlormadinone acetate, a few other progestins used in oral contraceptives and/or in menopausal HRT including dienogest, drospirenone, medrogestone, nomegestrol acetate, promegestone, and trimegestone also have varying degrees of AR antagonistic activity.[86][87][88]
- Androgen synthesis inhibitors: Drugs that directly inhibit the enzymatic biosynthesis of androgens like testosterone and/or DHT.[89][31] Examples include the CYP17A1 inhibitors ketoconazole, abiraterone acetate, and seviteronel,[89] the CYP11A1 (P450scc) inhibitor aminoglutethimide,[89] and the 5α-reductase inhibitors finasteride, dutasteride, epristeride, alfatradiol, and saw palmetto extract (Serenoa repens).[90] A number of other antiandrogens, including cyproterone acetate, spironolactone, medrogestone, flutamide, nilutamide, and bifluranol, are also known to weakly inhibit androgen synthesis.
- Antigonadotropins: Drugs that suppress the gonadotropin-releasing hormone (GnRH)-induced release of gonadotropins and consequent activation of gonadal androgen production.[2][91] Examples include GnRH modulators like leuprorelin (a GnRH agonist) and cetrorelix (a GnRH antagonist),[92] progestogens like allylestrenol, chlormadinone acetate, cyproterone acetate, gestonorone caproate, hydroxyprogesterone caproate, medroxyprogesterone acetate, megestrol acetate, osaterone acetate (veterinary), and oxendolone,[93][94] and estrogens like estradiol, estradiol esters, ethinylestradiol, conjugated estrogens, and diethylstilbestrol.[2][93]
- Miscellaneous: Drugs that oppose the effects of androgens by means other than the above. Examples include estrogens, especially oral and synthetic (e.g., ethinylestradiol, diethylstilbestrol), which stimulate sex hormone-binding globulin (SHBG) production in the liver and thereby decrease free and hence bioactive levels of testosterone and DHT; anticorticotropins such as glucocorticoids, which suppress the adrenocorticotropic hormone (ACTH)-induced production of adrenal androgens; and immunogens and vaccines against androstenedione like ovandrotone albumin and androstenedione albumin, which decrease levels of androgens via the generation of antibodies against the androgen and androgen precursor androstenedione (used only in veterinary medicine).
Certain antiandrogens combine multiple of the above mechanisms.[6][95] An example is the steroidal antiandrogen cyproterone acetate, which is a potent AR antagonist, a potent progestogen and hence antigonadotropin, a weak glucocorticoid and hence anticorticotropin, and a weak androgen synthesis inhibitor.[6][95][96][97]
| Abiraterone acetate | Steroidal | Androgen synthesis inhibitor | Zytiga | Oral | 2011 | Available | 523,000 |
| Allylestrenol | Steroidal | Progestin | Gestanin, Perselin | Oral | 1961 | Availableb | 61,800 |
| Aminoglutethimide | Nonsteroidal | Androgen synthesis inhibitor | Cytadren, Orimeten | Oral | 1960 | Availableb | 222,000 |
| Apalutamide | Nonsteroidal | AR antagonist | Erleada | Oral | 2018 | Available | 50,400 |
| Bicalutamide | Nonsteroidal | AR antagonist | Casodex | Oral | 1995 | Available | 754,000 |
| Chlormadinone acetate | Steroidal | Progestin; AR antagonist | Belara, Prostal | Oral | 1965 | Available | 220,000 |
| Cyproterone acetate | Steroidal | Progestin; AR antagonist | Androcur, Diane | Oral, IM | 1973 | Available | 461,000 |
| Delmadinone acetate | Steroidal | Progestin; AR antagonist | Tardak | Veterinary | 1972 | Veterinary | 42,600 |
| Enzalutamide | Nonsteroidal | AR antagonist | Xtandi | Oral | 2012 | Available | 328,000 |
| Flutamide | Nonsteroidal | AR antagonist | Eulexin | Oral | 1983 | Available | 712,000 |
| Gestonorone caproate | Steroidal | Progestin | Depostat, Primostat | IM | 1973 | Availableb | 119,000 |
| Hydroxyprogesterone caproate | Steroidal | Progestin | Delalutin, Proluton | IM | 1954 | Available | 108,000 |
| Ketoconazole | Nonsteroidal | Androgen synthesis inhibitor | Nizoral, others | Oral, topical | 1981 | Available | 3,650,000 |
| Medroxyprogesterone acetate | Steroidal | Progestin | Provera, Depo-Provera | Oral, IM, SC | 1958 | Available | 1,250,000 |
| Megestrol acetate | Steroidal | Progestin; AR antagonist | Megace | Oral | 1963 | Available | 253,000 |
| Nilutamide | Nonsteroidal | AR antagonist | Anandron, Nilandron | Oral | 1987 | Available | 132,000 |
| Osaterone acetate | Steroidal | Progestin; AR antagonist | Ypozane | Veterinary | 2007 | Veterinary | 87,600 |
| Oxendolone | Steroidal | Progestin; AR antagonist | Prostetin, Roxenone | IM | 1981 | Availableb | 36,100 |
| Spironolactone | Steroidal | AR antagonist | Aldactone | Oral, topical | 1959 | Available | 3,010,000 |
| Topilutamide and Finasteride | Nonsteroidal | AR antagonist | Eucapil | Topical | 2003 | Availableb | 36,300 |
| Footnotes: a = Hits = Google Search hits (as of February 2018). b = Availability limited / mostly discontinued. Class: Steroidal = Steroidal antiandrogen. Nonsteroidal = Nonsteroidal antiandrogen. Sources: See individual articles. | |||||||
Side effects
[edit]The side effects of antiandrogens vary depending on the type of antiandrogen – namely whether it is a selective AR antagonist or lowers androgen levels – as well as the presence of off-target activity in the antiandrogen in question.[21][98] For instance, whereas antigonadotropic antiandrogens like GnRH modulators and cyproterone acetate are associated with pronounced sexual dysfunction and osteoporosis in men, selective AR antagonists like bicalutamide are not associated with osteoporosis and have been associated with only minimal sexual dysfunction.[21][99][100] These differences are thought related to the fact that antigonadotropins suppress androgen levels and by extension levels of bioactive metabolites of androgens like estrogens and neurosteroids whereas selective AR antagonists similarly neutralize the effects of androgens but leave levels of androgens and hence their metabolites intact (and in fact can even increase them as a result of their progonadotropic effects).[21] As another example, the steroidal antiandrogens cyproterone acetate and spironolactone possess off-target actions including progestogenic, antimineralocorticoid, and/or glucocorticoid activity in addition to their antiandrogen activity, and these off-target activities can result in additional side effects.[98]
In males, the major side effects of antiandrogens are demasculinization and feminization.[101] These side effects include breast pain/tenderness and gynecomastia (breast development/enlargement), reduced body hair growth/density, decreased muscle mass and strength, feminine changes in fat mass and distribution, and reduced penile length and testicular size.[101] The rates of gynecomastia in men with selective AR antagonist monotherapy have been found to range from 30 to 85%.[102] In addition, antiandrogens can cause infertility, osteoporosis, hot flashes, sexual dysfunction (including loss of libido and erectile dysfunction), depression, fatigue, anemia, and decreased semen/ejaculate volume in males.[failed verification][101] Conversely, the side effects of selective AR antagonists in women are minimal.[80][103] However, antigonadotropic antiandrogens like cyproterone acetate can produce hypoestrogenism, amenorrhea, and osteoporosis in premenopausal women, among other side effects.[81][104][105] In addition, androgen receptor antagonists can produce unfavorable effects on cholesterol levels, which long-term may increase the risk of cardiovascular disease.[106][107][108][109][110][111][112]
A number of antiandrogens have been associated with hepatotoxicity.[113] These include, to varying extents, cyproterone acetate, flutamide, nilutamide, bicalutamide, aminoglutethimide, and ketoconazole.[113] In contrast, spironolactone, enzalutamide,[114] and other antiandrogens are not associated with significant rates of hepatotoxicity. However, although they do not pose a risk of hepatotoxicity, spironolactone has a risk of hyperkalemia and enzalutamide has a risk of seizures.[citation needed]
In women who are pregnant, antiandrogens can interfere with the androgen-mediated sexual differentiation of the genitalia and brain of male fetuses.[115] This manifests primarily as ambiguous genitalia – that is, undervirilized or feminized genitalia, which, anatomically, are a cross between a penis and a vagina – and theoretically also as femininity.[115][116] As such, antiandrogens are teratogens, and women who are pregnant should not be treated with an antiandrogen.[82] Moreover, women who can or may become pregnant are strongly recommended to take an antiandrogen only in combination with proper contraception.[82]
Overdose
[edit]Antiandrogens are relatively safe in acute overdose.[citation needed]
Interactions
[edit]Inhibitors and inducers of cytochrome P450 enzymes may interact with various antiandrogens.[citation needed]
Mechanism of action
[edit]Androgen receptor antagonists
[edit]| Antiandrogen | Relative binding affinities | ||||
|---|---|---|---|---|---|
| AR | PR | ER | GR | MR | |
| Cyproterone acetate | 8–10 | 60 | <0.1 | 5 | 1 |
| Chlormadinone acetate | 5 | 175 | <0.1 | 38 | 1 |
| Megestrol acetate | 5 | 152 | <0.1 | 50 | 3 |
| Spironolactone | 7 | 0.4a | <0.1 | 2a | 182 |
| Trimethyltrienolone | 3.6 | <1 | <1 | <1 | <1 |
| Inocoterone | 0.8 | <0.1 | <0.1 | <0.1 | <0.1 |
| Inocoterone acetate | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Flutamide | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Hydroxyflutamide | 0.5–0.8 | <0.1 | <0.1 | <0.1 | <0.1 |
| Nilutamide | 0.5–0.8 | <0.1 | <0.1 | <0.1 | <0.1 |
| Bicalutamide | 1.8 | <0.1 | <0.1 | <0.1 | <0.1 |
| Notes: (1): Reference ligands (100%) were testosterone for the AR, progesterone for the PR, estradiol for the ER, dexamethasone for the GR, and aldosterone for the MR. (2): Tissues were rat prostate (AR), rabbit uterus (PR), mouse uterus (ER), rat thymus (GR), and rat kidney (MR). (3): Incubation times (0 °C) were 24 hours (AR, a), 2 hours (PR, ER), 4 hours (GR), and 1 hour (MR). (4): Assay methods were different for bicalutamide for receptors besides the AR. Sources: [117][118][119][120][121][122][123][124][125][126] | |||||
| Antiandrogen | Relative potency |
|---|---|
| Bicalutamide | 4.3 |
| Hydroxyflutamide | 3.5 |
| Flutamide | 3.3 |
| Cyproterone acetate | 1.0 |
| Zanoterone | 0.4 |
| Description: Relative potencies of orally administered antiandrogens in antagonizing 0.8 to 1.0 mg/kg s.c. testosterone propionate-induced ventral prostate weight increase in castrated immature male rats. Higher values mean greater potency. Sources: See template. | |
AR antagonists act by directly binding to and competitively displacing androgens like testosterone and DHT from the AR, thereby preventing them from activating the receptor and mediating their biological effects.[84][85] AR antagonists are classified into two types, based on chemical structure: steroidal and nonsteroidal.[7][8][84][85][92] Steroidal AR antagonists are structurally related to steroid hormones like testosterone and progesterone, whereas nonsteroidal AR antagonists are not steroids and are structurally distinct. Steroidal AR antagonists tend to have off-target hormonal actions due to their structural similarity to other steroid hormones.[92] In contrast, nonsteroidal AR antagonists are selective for the AR and have no off-target hormonal activity.[92] For this reason, they are sometimes described as "pure" antiandrogens.[92]
Although they are described as antiandrogens and indeed show only such effects generally, most or all steroidal AR antagonists are actually not silent antagonists of the AR but rather are weak partial agonists and are able to activate the receptor in the absence of more potent AR agonists like testosterone and DHT.[84][31][127][128] This may have clinical implications in the specific context of prostate cancer treatment.[84][127] As an example, steroidal AR antagonists are able to increase prostate weight and accelerate prostate cancer cell growth in the absence of more potent AR agonists,[84][127] and spironolactone has been found to accelerate progression of prostate cancer in case reports.[129][130] In addition, whereas cyproterone acetate produces ambiguous genitalia via feminization in male fetuses when administered to pregnant animals,[131] it has been found to produce masculinization of the genitalia of female fetuses of pregnant animals.[84] In contrast to steroidal AR antagonists, nonsteroidal AR antagonists are silent antagonists of the AR and do not activate the receptor.[132][31][133][127] This may be why they have greater efficacy than steroidal AR antagonists in the treatment of prostate cancer and is an important reason as to why they have largely replaced them for this indication in medicine.[132][31][133][127]
Nonsteroidal antiandrogens have relatively low affinity for the AR compared to steroidal AR ligands.[31][133][134] For example, bicalutamide has around 2% of the affinity of DHT for the AR and around 20% of the affinity of CPA for the AR.[134] Despite their low affinity for the AR however, the lack of weak partial agonist activity of NSAAs appears to improve their potency relative to steroidal antiandrogens.[134][135] For example, although flutamide has about 10-fold lower affinity for the AR than CPA, it shows equal or slightly greater potency to CPA as an antiandrogen in bioassays.[134][135] In addition, circulating therapeutic concentrations of nonsteroidal antiandrogens are very high, on the order of thousands of times higher than those of testosterone and DHT, and this allows them to efficaciously compete and block AR signaling.[136]
AR antagonists may not bind to or block membrane androgen receptors (mARs), which are distinct from the classical nuclear AR.[137][138][139] However, the mARs do not appear to be involved in masculinization. This is evidenced by the perfectly female phenotype of women with complete androgen insensitivity syndrome.[140][141] These women have a 46,XY karyotype (i.e., are genetically "male") and high levels of androgens but possess a defective AR and for this reason never masculinize.[140][141] They are described as highly feminine, both physically as well as mentally and behaviorally.[142][143][144]
N-Terminal domain antagonists
[edit]N-Terminal domain AR antagonists are a new type of AR antagonist that, unlike all currently marketed AR antagonists, bind to the N-terminal domain (NTD) of the AR rather than the ligand-binding domain (LBD).[145] Whereas conventional AR antagonists bind to the LBD of the AR and competitively displace androgens, thereby preventing them from activating the receptor, AR NTD antagonists bind covalently to the NTD of the AR and prevent protein–protein interactions subsequent to activation that are required for transcriptional activity.[145] As such, they are non-competitive and irreversible antagonists of the AR.[146] Examples of AR NTD antagonists include bisphenol A diglycidyl ether (BADGE) and its derivatives EPI-001, ralaniten (EPI-002), and ralaniten acetate (EPI-506).[145][147] AR NTD antagonists are under investigation for the potential treatment of prostate cancer, and it is thought that they may have greater efficacy as antiandrogens relative to conventional AR antagonists.[145] In accordance with this notion, AR NTD antagonists are active against splice variants of the AR, which conventional AR antagonists are not, and AR NTD antagonists are immune to gain-of-function mutations in the AR LBD that convert AR antagonists into AR agonists and commonly occur in prostate cancer.[145]
Androgen receptor degraders
[edit]Selective androgen receptor degraders (SARDs) are another new type of antiandrogen that has recently been developed.[148] They work by enhancing the degradation of the AR, and are analogous to selective estrogen receptor degraders (SERDs) like fulvestrant (a drug used to treat estrogen receptor-positive breast cancer).[148] Similarly to AR NTD antagonists, it is thought that SARDs may have greater efficacy than conventional AR antagonists, and for this reason, they are under investigation for the treatment of prostate cancer.[149] An example of a SARD is dimethylcurcumin (ASC-J9), which is under development as a topical medication for the potential treatment of acne.[150] SARDs like dimethylcurcumin differ from conventional AR antagonists and AR NTD antagonists in that they may not necessarily bind directly to the AR.[149]
Androgen synthesis inhibitors
[edit]Androgen synthesis inhibitors are enzyme inhibitors that prevent the biosynthesis of androgens.[31] This process occurs mainly in the gonads and adrenal glands, but also occurs in other tissues like the prostate gland, skin, and hair follicles. These drugs include aminoglutethimide, ketoconazole,[151] and abiraterone acetate.[89][31][152] Aminoglutethimide inhibits cholesterol side-chain cleavage enzyme, also known as P450scc or CYP11A1, which is responsible for the conversion of cholesterol into pregnenolone and by extension the production of all steroid hormones, including the androgens.[89] Ketoconazole and abiraterone acetate are inhibitors of the enzyme CYP17A1, also known as 17α-hydroxylase/17,20-lyase, which is responsible for the conversion of pregnane steroids into androgens, as well as the conversion of mineralocorticoids into glucocorticoids.[89][31] Because these drugs all prevent the formation of glucocorticoids in addition to androgens, they must be combined with a glucocorticoid like prednisone to avoid adrenal insufficiency.[152] A newer drug currently under development for treatment of prostate cancer, seviteronel, is selective for inhibition of the 17,20-lyase functionality of CYP17A1, and for this reason, unlike earlier drugs, does not require concomitant treatment with a glucocorticoid.[153]
5α-Reductase inhibitors
[edit]5α-Reductase inhibitors such as finasteride and dutasteride are inhibitors of 5α-reductase, an enzyme that is responsible for the formation of DHT from testosterone.[154] DHT is between 2.5- and 10-fold more potent than testosterone as an androgen[155] and is produced in a tissue-selective manner based on expression of 5α-reductase.[156] Tissues in which DHT forms at a high rate include the prostate gland, skin, and hair follicles.[41][156] In accordance, DHT is involved in the pathophysiology of benign prostatic hyperplasia, pattern hair loss, and hirsutism, and 5α-reductase inhibitors are used to treat these conditions.[41][156][157]
Antigonadotropins
[edit]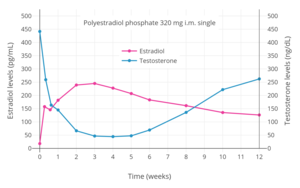

Antigonadotropins are drugs that suppress the GnRH-mediated secretion of gonadotropins from the pituitary gland.[91] Gonadotropins include luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and are peptide hormones that signal the gonads to produce sex hormones. By suppressing gonadotropin secretion, antigonadotropins suppress gonadal sex hormone production and by extension circulating androgen levels.[91] GnRH modulators, including both GnRH agonists and GnRH antagonists, are powerful antigonadotropins that are able to suppress androgen levels by 95% in men.[160] In addition, estrogens and progestogens are antigonadotropins via exertion of negative feedback on the hypothalamic–pituitary–gonadal axis (HPG axis).[2][93][161] High-dose estrogens are able to suppress androgen levels to castrate levels in men similarly to GnRH modulators,[162] while high-dose progestogens are able to suppress androgen levels by up to approximately 70 to 80% in men.[163][164]
Examples of GnRH agonists include leuprorelin (leuprolide) and goserelin, while an example of a GnRH antagonist is cetrorelix.[92] Estrogens that are or that have been used as antigonadotropins include estradiol, estradiol esters like estradiol valerate, estradiol undecylate, and polyestradiol phosphate, conjugated estrogens, ethinylestradiol, diethylstilbestrol (no longer widely used), and bifluranol.[165][166] Progestogens that are used as antigonadotropins include chlormadinone acetate, cyproterone acetate, gestonorone caproate,[167] hydroxyprogesterone caproate, medroxyprogesterone acetate, megestrol acetate, and oxendolone.[2][168][169]
Miscellaneous
[edit]Sex hormone-binding globulin modulators
[edit]In addition to their antigonadotropic effects, estrogens are also functional antiandrogens by decreasing free concentrations of androgens via increasing the hepatic production of sex hormone-binding globulin (SHBG) and by extension circulating SHBG levels.[170][171][172] Combined oral contraceptives containing ethinylestradiol have been found to increase circulating SHBG levels by 2- to 4-fold in women and to reduce free testosterone concentrations by 40 to 80%.[171] However, combined oral contraceptives that contain the particularly androgenic progestin levonorgestrel have been found to increase SHBG levels by only 50 to 100%,[171] which is likely because activation of the AR in the liver has the opposite effect of estrogen and suppresses production of SHBG.[173] Levonorgestrel and certain other 19-nortestosterone progestins used in combined oral contraceptives like norethisterone also directly bind to and displace androgens from SHBG, which may additionally antagonize the functional antiandrogenic effects of ethinylestradiol.[173][174] In men, a study found that treatment with a relatively low dosage of 20 μg/day ethinylestradiol for 5 weeks increased circulating SHBG levels by 150% and, due to the accompanying decrease free testosterone levels, increased total circulating levels of testosterone by 50% (via reduced negative feedback by androgens on the HPG axis).[170]
Corticosteroid-binding globulin modulators
[edit]Estrogens at high doses can partially suppress adrenal androgen production.[175][176][177][178][179][180] A study found that treatment with a high-dose ethinylestradiol (100 μg/day) reduced levels of major circulating adrenal androgens by 27 to 48% in transgender women.[175][176][177] Decreased adrenal androgens with estrogens is apparent with oral and synthetic estrogens like ethinylestradiol and estramustine phosphate but is minimal with parenteral bioidentical estradiol forms like polyestradiol phosphate.[179] It is thought to be mediated via a hepatic mechanism, probably increased corticosteroid-binding globulin (CBG) production and levels and compensatory changes in adrenal steroid production (e.g., shunting of adrenal androgen synthesis to cortisol production).[179][180] It is notable in this regard that oral and synthetic estrogens, due to the oral first pass and resistance to hepatic metabolism, have much stronger influences on liver protein synthesis than parenteral estradiol.[181] The decrease in adrenal androgen levels with high-dose estrogen therapy may be beneficial in the treatment of prostate cancer.[177][180]
Anticorticotropins
[edit]Anticorticotropins such as glucocorticoids and mineralocorticoids work by exerting negative feedback on the hypothalamic–pituitary–adrenal axis (HPA axis), thereby inhibiting the secretion of corticotropin-releasing hormone (CRH) and hence adrenocorticotropic hormone (ACTH; corticotropin) and consequently suppressing the production of androgen prohormones like dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and androstenedione in the adrenal gland.[182][183] They are rarely used clinically as functional antiandrogens, but are used as such in the case of congenital adrenal hyperplasia in girls and women, in which there are excessive production and levels of adrenal androgens due to glucocorticoid deficiency and hence HPA axis overactivity.[182][183]
Insulin sensitizers
[edit]In women with insulin resistance, such as those with polycystic ovary syndrome, androgen levels are often elevated.[184] Metformin, an insulin-sensitizing medication, has indirect antiandrogenic effects in such women, decreasing testosterone levels by as much as 50% secondary to its beneficial effects on insulin sensitivity.[184][185][186]
Immunogens and vaccines
[edit]Ovandrotone albumin (Fecundin, Ovastim) and Androvax (androstenedione albumin) are immunogens and vaccines against androstenedione that are used in veterinary medicine to improve fecundity (reproductive rate) in ewes (adult female sheep).[187][188] The generation of antibodies against androstenedione by these agents is thought to decrease circulating levels of androstenedione and its metabolites (e.g., testosterone and estrogens), which in turn increases the activity of the HPG axis via reduced negative feedback and increases the rate of ovulation, resulting in greater fertility and fecundity.[187][188]
Chemistry
[edit]Antiandrogens can be divided into several different types based on chemical structure, including steroidal antiandrogens, nonsteroidal antiandrogens, and peptides. Steroidal antiandrogens include compounds like cyproterone acetate, spironolactone, estradiol, abiraterone acetate, and finasteride; nonsteroidal antiandrogens include compounds like bicalutamide, elagolix, diethylstilbestrol, aminoglutethimide, and ketoconazole; and peptides include GnRH analogues like leuprorelin and cetrorelix.[citation needed]
History
[edit]Antigonadotropins like estrogens and progestogens were both first introduced in the 1930s.[189] The beneficial effects of androgen deprivation via surgical castration or high-dose estrogen therapy on prostate cancer were discovered in 1941.[31]: 56 [190] AR antagonists were first discovered in the early 1960s.[96] The steroidal antiandrogen cyproterone acetate was discovered in 1961. and introduced in 1973. and is often described as the first antiandrogen to have been marketed.[191][62] However, spironolactone was introduced in 1959.,[192][193] although its antiandrogen effects were not recognized or taken advantage of until later and were originally an unintended off-target action of the drug.[194] In addition to spironolactone, chlormadinone acetate and megestrol acetate are steroidal antiandrogens that are weaker than cyproterone acetate but were also introduced earlier, in the 1960s.[195][196][197] Other early steroidal antiandrogens that were developed around this time but were never marketed include benorterone (SKF-7690; 17α-methyl-B-nortestosterone), BOMT (Ro 7–2340), cyproterone (SH-80881), and trimethyltrienolone (R-2956).[198][199]
The nonsteroidal antiandrogen flutamide was first reported in 1967.[24] It was introduced in 1983 and was the first nonsteroidal antiandrogen marketed.[200][201] Another early nonsteroidal antiandrogen,[202] DIMP (Ro 7–8117), which is structurally related to thalidomide[203] and is a relatively weak antiandrogen,[204][205] was first described in 1973 and was never marketed.[206] Flutamide was followed by nilutamide in 1989. and bicalutamide in 1995.[207] In addition to these three drugs, which have been regarded as first-generation nonsteroidal antiandrogens, the second-generation nonsteroidal antiandrogens enzalutamide and apalutamide were introduced in 2012. and 2018.[208][209][210] They differ from the earlier nonsteroidal antiandrogens namely in that they are much more efficacious in comparison.[209]
The androgen synthesis inhibitors aminoglutethimide and ketoconazole were first marketed in 1960. and 1977., respectively,[211][212] and the newer drug abiraterone acetate was introduced in 2011.[213] GnRH modulators were first introduced in the 1980s.[214] The 5α-reductase inhibitors finasteride and dutasteride were introduced in 1992. and 2002. respectively.[215][216] Elagolix, the first orally active GnRH modulator to be marketed, was introduced in 2018.[217]
Timeline
[edit]The following is a timeline of events in the history of antiandrogens:[218]
- 1941: Hudgins and Hodges show that androgen deprivation via high-dose estrogen therapy or surgical castration treats prostate cancer
- 1957: The steroidal antiandrogen spironolactone is first synthesized[219]
- 1960: Spironolactone is first introduced for medical use, as an antimineralocorticoid[219]
- 1961: The steroidal antiandrogen cyproterone acetate is first synthesized[220]
- 1962: Spironolactone is first reported to produce gynecomastia in men[219][221]
- 1966: Benorterone is the first known antiandrogen to be studied clinically, to treat acne and hirsutism in women[222][223]
- 1963: The antiandrogenic activity of cyproterone acetate is discovered[48][224]
- 1967: A known antiandrogen, benorterone, is first reported to induce gynecomastia in males[222]
- 1967: The first-generation nonsteroidal antiandrogen flutamide is first synthesized
- 1967: Cyproterone acetate was first studied clinically, to treat sexual deviance in men[225]
- 1969: Cyproterone acetate was first studied in the treatment of acne, hirsutism, seborrhea, and scalp hair loss in women[226]
- 1969: The antiandrogenic activity of spironolactone is discovered[227]
- 1972: The antiandrogenic activity of flutamide is first reported[228][229]
- 1973: Cyproterone acetate was first introduced for medical use, to treat sexual deviance[230]
- 1977: The first-generation antiandrogen nilutamide is first described[231]
- 1978: Spironolactone is first studied in the treatment of hirsutism in women[65][232]
- 1979: Combined androgen blockade is first studied[233][234]
- 1980: Medical castration via a GnRH analogue is first achieved[citation needed]
- 1982: The first-generation antiandrogen bicalutamide is first described[235]
- 1982: Combined androgen blockade for prostate cancer is developed
- 1983: Flutamide is first introduced, in Chile, for medical use, to treat prostate cancer[236][237]
- 1987: Nilutamide is first introduced, in France, for medical use, to treat prostate cancer[207]
- 1989: Combined androgen blockade via flutamide and a GnRH analogue is found to be superior to a GnRH analogue alone for prostate cancer
- 1989: Flutamide is first introduced for medical use in the United States, to treat prostate cancer[238]
- 1989: Flutamide is first studied in the treatment of hirsutism in women[9]
- 1992: The androgen synthesis inhibitor abiraterone acetate is first described[239]
- 1995: Bicalutamide is first introduced for medical use, to treat prostate cancer[207]
- 1996: Nilutamide is first introduced for medical use in the United States, to treat prostate cancer[240]
- 2006: The second-generation nonsteroidal antiandrogen enzalutamide is first described[241]
- 2007: The second-generation nonsteroidal antiandrogen apalutamide is first described[242]
- 2011: Abiraterone acetate is first introduced for medical use, to treat prostate cancer[243]
- 2012: Enzalutamide is first introduced for medical use, to treat prostate cancer[244]
- 2018: Apalutamide is first introduced for medical use, to treat prostate cancer[245]
- 2018: Elagolix is the first orally active GnRH antagonist to be introduced for medical use[217]
- 2019: Relugolix is the second orally active GnRH antagonist to be introduced for medical use[246]
Society and culture
[edit]Etymology
[edit]The term antiandrogen is generally used to refer specifically to AR antagonists, as described by Dorfman (1970):[247][248]
Antiandrogens are substances which prevent androgens from expressing their activity at target sites. The inhibitory effect of these substances, therefore, should be differentiated from compounds which decrease the synthesis and/or release of hypothalamic (releasing) factors, from anterior pituitary hormones (gonadotropins, particularly luteinizing hormone) and from material which acts directly on the gonads to inhibit biosynthesis and/or secretion of androgens.[247][248]
However, in spite of the above, the term may also be used to describe functional antiandrogens like androgen synthesis inhibitors and antigonadotropins, including even estrogens and progestogens.[2][6][249] For example, the progestogen and hence antigonadotropin medroxyprogesterone acetate is sometimes described as a steroidal antiandrogen, even though it is not an antagonist of the AR.[250][249]
Research
[edit]Topical administration
[edit]This section's factual accuracy may be compromised due to out-of-date information. The reason given is: Clascoterone has been approved for acne by the FDA in August 2020, and is considered to be highly effective (>= tretinoin 0.05%). (April 2024) |
There has been much interest and effort in the development of topical AR antagonists to treat androgen-dependent conditions like acne and pattern hair loss in males.[251] Unfortunately, whereas systemic administration of antiandrogens is very effective in treating these conditions, topical administration has disappointingly been found generally to possess limited and only modest effectiveness, even when high-affinity steroidal AR antagonists like cyproterone acetate and spironolactone have been employed.[251] Moreover, in the specific case of acne treatment, topical AR antagonists have been found much less effective compared to established treatments like benzoyl peroxide and antibiotics.[251]
A variety of AR antagonists have been developed for topical use but have not completed development and hence have never been marketed. These include the steroidal AR antagonists clascoterone, cyproterone, rosterolone, and topterone and the nonsteroidal AR antagonists cioteronel, inocoterone acetate, RU-22930, RU-58642, and RU-58841. However, one topical AR antagonist, topilutamide (fluridil), has been introduced in a few European countries for the treatment of pattern hair loss in men.[40] In addition, a topical 5α-reductase inhibitor and weak estrogen, alfatradiol, has also been introduced in some European countries for the same indication, although its effectiveness is controversial.[40] Spironolactone has been marketed in Italy in the form of a topical cream under the brand name Spiroderm for the treatment of acne and hirsutism, but this formulation was discontinued and hence is no longer available.[252]
Male contraception
[edit]Antiandrogens, such as cyproterone acetate, have been studied for potential use as male hormonal contraceptives.[253][254][255][256][65][257][258][259] While effective in suppressing male fertility, their use as monotherapies is precluded by side effects, such as androgen deficiency (e.g., demasculinization, sexual dysfunction, hot flashes, osteoporosis) and feminization (e.g., gynecomastia).[65][257][258][260] The combination of a primary antigonadotropin such as cyproterone acetate to prevent fertility and an androgen like testosterone to prevent systemic androgen deficiency, resulting in a selective antiandrogenic action locally in the testes, has been extensively studied and has shown promising results, but has not been approved for clinical use at this time.[258][259][261][262][260] Dimethandrolone undecanoate (developmental code name CDB-4521), an orally active dual AAS and progestogen, is under investigation as a potential male contraceptive and as the first male birth control pill.[263][264]
Breast cancer
[edit]Antiandrogens such as bicalutamide, enzalutamide, and abiraterone acetate are under investigation for the potential treatment of breast cancer, including AR-expressing triple-negative breast cancer and other types of AR-expressing breast cancer.[265][266][267][268][269]
Miscellaneous
[edit]Antiandrogens may be effective in the treatment of obsessive–compulsive disorder.[270]
See also
[edit]References
[edit]- ^ Mowszowicz I (1989). "Antiandrogens. Mechanisms and paradoxical effects". Ann. Endocrinol. 50 (3). Paris: 50(3):189–99. PMID 2530930.
- ^ a b c d e f Brueggemeier RW (2006). "Sex Hormones (Male): Analogs and Antagonists". Encyclopedia of Molecular Cell Biology and Molecular Medicine. Wiley-VCH Verlag GmbH & Co. KGaA. doi:10.1002/3527600906.mcb.200500066. ISBN 3527600906.
- ^ Nath JL (2006). Using Medical Terminology: A Practical Approach. Lippincott Williams & Wilkins. pp. 977–. ISBN 978-0-7817-4868-1.
- ^ a b c d e Student S, Hejmo T, Poterała-Hejmo A, Leśniak A, Bułdak R (January 2020). "Anti-androgen hormonal therapy for cancer and other diseases". Eur. J. Pharmacol. 866: 172783. doi:10.1016/j.ejphar.2019.172783. PMID 31712062.
- ^ Gillatt D (2006). "Antiandrogen treatments in locally advanced prostate cancer: are they all the same?". J Cancer Res Clin Oncol. 1: S17-26. doi:10.1007/s00432-006-0133-5. PMID 16845534. S2CID 23888640.
- ^ a b c d e f g h Lieberman R (2001). "Androgen deprivation therapy for prostate cancer chemoprevention: current status and future directions for agent development". Urology. 58 (2 Suppl 1): 83–90. doi:10.1016/s0090-4295(01)01247-x. PMID 11502457.
There are several classes of antiandrogens including (1) antigonadotropins (eg, LHRH agonists/antagonists, synthetic estrogens [diethylstilbestrol]); (2) nonsteroidal androgen-receptor antagonists (eg, flutamide, bicalutamide, nilutamide); (3) steroidal agents with mixed actions (eg, cyproterone acetate); (4) adrenal androgen inhibitors (eg, ketoconazole, hydrocortisone); (5) steroidal agents that inhibit androgen biosynthesis (eg, 5α-reductase inhibitors (type II) and dual-acting 5α-reductase inhibitors); [...]
- ^ a b c Schröder FH, Radlmaier A (2009). "Steroidal Antiandrogens". In Jordan VC, Furr BA (eds.). Hormone Therapy in Breast and Prostate Cancer. Humana Press. pp. 325–346. doi:10.1007/978-1-59259-152-7_15. ISBN 978-1-60761-471-5.
- ^ a b c Kolvenbag GJ, Furr BJ (2009). "Nonsteroidal Antiandrogens". In Jordan VC, Furr BJ (eds.). Hormone Therapy in Breast and Prostate Cancer. Humana Press. pp. 347–368. doi:10.1007/978-1-59259-152-7_16. ISBN 978-1-60761-471-5.
- ^ a b c d Sciarra F, Toscano V, Concolino G, Di Silverio F (November 1990). "Antiandrogens: clinical applications". The Journal of Steroid Biochemistry and Molecular Biology. 37 (3): 349–362. doi:10.1016/0960-0760(90)90484-3. PMID 2147859. S2CID 20274398.
- ^ Broderick GA, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R (January 2010). "Priapism: pathogenesis, epidemiology, and management". The Journal of Sexual Medicine. 7 (1 Pt 2): 476–500. doi:10.1111/j.1743-6109.2009.01625.x. PMID 20092449.
- ^ Steinberg MH, Forget BG, Higgs DR, Weatherall DJ (17 August 2009). Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge University Press. pp. 476–. ISBN 978-1-139-48080-2.
- ^ a b c Essah PA, Wickham EP, Nunley JR, Nestler JE (2006). "Dermatology of androgen-related disorders". Clinics in Dermatology. 24 (4): 289–298. doi:10.1016/j.clindermatol.2006.04.004. PMID 16828411.
- ^ a b c d e f g h Rabe T, Grunwald K, Feldmann K, Runnebaum B (2009). "Treatment of hyperandrogenism in women". Gynecological Endocrinology. 10 (sup3): 1–44. doi:10.3109/09513599609045658. ISSN 0951-3590.
- ^ a b c d Bockting W, Coleman E, De Cuypere G (2011). "Care of transsexual persons". N. Engl. J. Med. 364 (26): 2559–60, author reply 2560. doi:10.1056/NEJMc1104884. PMID 21714669.
- ^ a b Vance SR, Ehrensaft D, Rosenthal SM (2014). "Psychological and medical care of gender nonconforming youth" (PDF). Pediatrics. 134 (6): 1184–92. doi:10.1542/peds.2014-0772. PMID 25404716. S2CID 5743822. Archived (PDF) from the original on 2018-05-15. Retrieved 2018-05-14.
- ^ a b c d e f g h Wadosky KM, Koochekpour S (2016). "Therapeutic Rationales, Progresses, Failures, and Future Directions for Advanced Prostate Cancer". Int. J. Biol. Sci. 12 (4): 409–26. doi:10.7150/ijbs.14090. PMC 4807161. PMID 27019626.
- ^ a b Massard C, Fizazi K (2011). "Targeting continued androgen receptor signaling in prostate cancer". Clin. Cancer Res. 17 (12): 3876–83. doi:10.1158/1078-0432.CCR-10-2815. PMID 21680543.
- ^ Msaouel P, Diamanti E, Tzanela M, Koutsilieris M (2007). "Luteinising hormone-releasing hormone antagonists in prostate cancer therapy". Expert Opin Emerg Drugs. 12 (2): 285–99. doi:10.1517/14728214.12.2.285. PMID 17604502. S2CID 41988320.
- ^ a b Akaza H (Jan 2011). "Combined androgen blockade for prostate cancer: review of efficacy, safety, and cost-effectiveness". Cancer Science. 102 (1): 51–6. doi:10.1111/j.1349-7006.2010.01774.x. PMID 21091846. S2CID 38486547.
- ^ Mateo J, Smith A, Ong M, de Bono JS (2014). "Novel drugs targeting the androgen receptor pathway in prostate cancer". Cancer Metastasis Rev. 33 (2–3): 567–79. doi:10.1007/s10555-013-9472-2. PMID 24390422. S2CID 13980764.
- ^ a b c d Iversen P, Melezinek I, Schmidt A (2001). "Nonsteroidal antiandrogens: a therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function". BJU Int. 87 (1): 47–56. doi:10.1046/j.1464-410x.2001.00988.x. PMID 11121992. S2CID 28215804.
- ^ Kolvenbag GJ, Iversen P, Newling DW (August 2001). "Antiandrogen monotherapy: a new form of treatment for patients with prostate cancer". Urology. 58 (2 Suppl 1): 16–23. doi:10.1016/s0090-4295(01)01237-7. PMID 11502439.
- ^ Mcleod DG (2003). "Hormonal therapy: historical perspective to future directions". Urology. 61 (2 Suppl 1): 3–7. doi:10.1016/s0090-4295(02)02393-2. PMID 12667881.
- ^ a b Smith HJ, Williams H (10 October 2005). Smith and Williams' Introduction to the Principles of Drug Design and Action, Fourth Edition. CRC Press. pp. 489–. ISBN 978-0-203-30415-0.
- ^ Chabner BA, Longo DL (8 November 2010). Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott Williams & Wilkins. pp. 679–680. ISBN 978-1-60547-431-1. Archived from the original on 10 January 2023. Retrieved 27 December 2016.
From a structural standpoint, antiandrogens are classified as steroidal, including cyproterone [acetate] (Androcur) and megestrol [acetate], or nonsteroidal, including flutamide (Eulexin, others), bicalutamide (Casodex), and nilutamide (Nilandron). The steroidal antiandrogens are rarely used.
- ^ Kaliks RA, Del Giglio A (2008). "Management of advanced prostate cancer" (PDF). Revista da Associação Médica Brasileira. 54 (2): 178–82. doi:10.1590/S0104-42302008000200025. PMID 18506331. Archived (PDF) from the original on 2017-05-10. Retrieved 2016-12-27.
- ^ Chang S (10 March 2010), Bicalutamide BPCA Drug Use Review in the Pediatric Population (PDF), U.S. Department of Health and Human Service, archived (PDF) from the original on 24 October 2016, retrieved 20 July 2016
- ^ Gulley JL (2011). Prostate Cancer. Demos Medical Publishing. pp. 81–. ISBN 978-1-935281-91-7.
- ^ Lutz M (1 January 2008). Controversies in the Treatment of Prostate Cancer. Karger Medical and Scientific Publishers. pp. 41–42. ISBN 978-3-8055-8524-8. Archived from the original on 12 January 2023. Retrieved 27 December 2016.
- ^ Prostate Cancer. Demos Medical Publishing. 20 December 2011. pp. 505–. ISBN 978-1-935281-91-7.
- ^ a b c d e f g h i j Figg W, Chau CH, Small EJ (14 September 2010). Drug Management of Prostate Cancer. Springer Science & Business Media. pp. 71–72, 75, 91–96. ISBN 978-1-60327-829-4.
- ^ a b Rittmaster RS (2011). "Chemoprevention of prostate cancer". Acta Oncol. 50 (Suppl 1): 127–36. doi:10.3109/0284186X.2010.527367. PMID 21604953.
- ^ Mackenzie IS, Morant SV, Wei L, Thompson AM, MacDonald TM (2016). "Spironolactone use and risk of incident cancers: a retrospective, matched cohort study". Br J Clin Pharmacol. 83 (3): 653–663. doi:10.1111/bcp.13152. PMC 5306481. PMID 27735065.
- ^ Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ, Spack NP, Tangpricha V, Montori VM (2009). "Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline". J. Clin. Endocrinol. Metab. 94 (9): 3132–54. doi:10.1210/jc.2009-0345. PMID 19509099.
- ^ Gooren L, Morgentaler A (2014). "Prostate cancer incidence in orchidectomised male-to-female transsexual persons treated with oestrogens". Andrologia. 46 (10): 1156–60. doi:10.1111/and.12208. PMID 24329588. S2CID 1445627.
- ^ Turo R, Jallad S, Prescott S, Cross WR (2013). "Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy". Can Urol Assoc J. 7 (7–8): E544–6. doi:10.5489/cuaj.175. PMC 3758950. PMID 24032068.
- ^ a b Dörsam J, Altwein J (2009). "5alpha-Reductase inhibitor treatment of prostatic diseases: background and practical implications". Prostate Cancer Prostatic Dis. 12 (2): 130–6. doi:10.1038/pcan.2008.56. PMID 19030020.
- ^ Ishizuka O, Nishizawa O, Hirao Y, Ohshima S (2002). "Evidence-based meta-analysis of pharmacotherapy for benign prostatic hypertrophy". Int. J. Urol. 9 (11): 607–12. doi:10.1046/j.1442-2042.2002.00539.x. PMID 12534901. S2CID 8249363.
- ^ Raspé G, Brosig W (22 October 2013). International Symposium on the Treatment of Carcinoma of the Prostate, Berlin, November 13 to 15, 1969: Life Science Monographs. Elsevier. pp. 165–. ISBN 978-1-4831-8711-2.
- ^ a b c Trüeb RM, Lee WS (13 February 2014). Male Alopecia: Guide to Successful Management. Springer Science & Business Media. pp. 91–93. ISBN 978-3-319-03233-7.
- ^ a b c Bolognia JL, Jorizzo JL, Rapini RP (2003). Dermatology. Gulf Professional Publishing. pp. 1072–. ISBN 9789997638991.
- ^ a b Simpson NB (1989). "The Effect of Drugs on Hair". Pharmacology of the Skin II. Handbook of Experimental Pharmacology. Vol. 87 / 2. Springer. pp. 495–508. doi:10.1007/978-3-642-74054-1_37. ISBN 978-3-642-74056-5. ISSN 0171-2004.
- ^ a b Unger WP (1 February 1995). "Androgenetic alopecia and its treatment. A historical overview". Hair Transplantation (Third ed.). Taylor & Francis. pp. 1–33. ISBN 978-0-8247-9363-0.
- ^ a b Rasmusson GH (1986). Chapter 18. Chemical Control of Androgen Action. Annual Reports in Medicinal Chemistry. Vol. 21. Academic Press. pp. 179–188. doi:10.1016/S0065-7743(08)61128-8. ISBN 9780120405213. ISSN 0065-7743.
- ^ a b Cormane RH, van der Meeren HL (1981). "Cyproteronacetate in the management of severe acne in males". Archives of Dermatological Research. 271 (2): 183–187. doi:10.1007/BF00412545. ISSN 0340-3696. S2CID 12153042.
- ^ Giltay EJ, Gooren LJ (2009). "Potential side effects of androgen deprivation treatment in sex offenders". The Journal of the American Academy of Psychiatry and the Law. 37 (1): 53–58. PMID 19297634.
- ^ Lam SM, Hempstead BR, Williams EF (2012). "Medical Management Options for Hair Loss". Aesthetic Medicine. Springer. pp. 529–535. doi:10.1007/978-3-642-20113-4_41. ISBN 978-3-642-20112-7.
- ^ a b Neumann F (1996). "Pharmacology of Cyproterone Acetate — A Short Review". Antiandrogens in Prostate Cancer. pp. 31–44. doi:10.1007/978-3-642-45745-6_3. ISBN 978-3-642-45747-0.
- ^ Coskey RJ (July 1984). "Dermatologic therapy: December, 1982, through November, 1983". Journal of the American Academy of Dermatology. 11 (1): 25–52. doi:10.1016/S0190-9622(84)80163-2. PMID 6376557.
- ^ Plewig G, Kligman AM (6 December 2012). ACNE and ROSACEA. Springer Science & Business Media. pp. 687–. ISBN 978-3-642-59715-2.
- ^ Alldredge BK, Corelli RL, Ernst ME (1 February 2012). Koda-Kimble and Young's Applied Therapeutics: The Clinical Use of Drugs. Lippincott Williams & Wilkins. pp. 952–. ISBN 978-1-60913-713-7.
- ^ Ward A, Brogden RN, Heel RC, Speight TM, Avery GS (July 1984). "Isotretinoin. A review of its pharmacological properties and therapeutic efficacy in acne and other skin disorders". Drugs. 28 (1): 6–37. doi:10.2165/00003495-198428010-00002. PMID 6235105.
- ^ Misch KJ, Dolman WF, Neild V, Rhodes EL (1986). "Response of male acne to antiandrogen therapy with cyproterone acetate". Dermatologica. 173 (3): 139–142. doi:10.1159/000249236. PMID 2945742.
- ^ Kircik LH (July 2019). "What's new in the management of acne vulgaris". Cutis. 104 (1): 48–52. PMID 31487336. Archived from the original on 2020-10-26. Retrieved 2020-03-30.
- ^ Hassoun LA, Chahal DS, Sivamani RK, Larsen LN (June 2016). "The use of hormonal agents in the treatment of acne". Seminars in Cutaneous Medicine and Surgery. 35 (2): 68–73. doi:10.12788/j.sder.2016.027 (inactive 1 November 2024). PMID 27416311.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ "Cassiopea Receives FDA Approval for Winlevi® (clascoterone cream 1%), First-in-Class Topical Acne Treatment Targeting the Androgen Receptor - Cassiopea". 2020-08-28. Archived from the original on 2020-08-28. Retrieved 2024-04-15.
- ^ "Drugs@FDA: FDA-Approved Drugs". www.accessdata.fda.gov. Retrieved 2024-04-15.
- ^ Jones RE, Lopez KH (28 September 2013). Human Reproductive Biology. Academic Press. pp. 77–. ISBN 978-0-12-382185-0.
- ^ a b Bradford JM (2001). "The neurobiology, neuropharmacology, and pharmacological treatment of the paraphilias and compulsive sexual behaviour". Can J Psychiatry. 46 (1): 26–34. doi:10.1177/070674370104600104. PMID 11221487.
- ^ a b Guay DR (2009). "Drug treatment of paraphilic and nonparaphilic sexual disorders". Clin Ther. 31 (1): 1–31. doi:10.1016/j.clinthera.2009.01.009. PMID 19243704.
- ^ Marshall WL, Laws DR, Barbaree HE (21 November 2013). Handbook of Sexual Assault: Issues, Theories, and Treatment of the Offender. Springer Science & Business Media. pp. 297–. ISBN 978-1-4899-0915-2.
- ^ a b Stunkard AJ, Baum A (1989). Eating, Sleeping, and Sex. Psychology Press. pp. 209–. ISBN 978-0-8058-0280-1.
- ^ Phenix A, Hoberman HM (7 December 2015). Sexual Offending: Predisposing Antecedents, Assessments and Management. Springer. pp. 759–. ISBN 978-1-4939-2416-5.
- ^ a b c d Brito VN, Latronico AC, Arnhold IJ, Mendonça BB (February 2008). "Update on the etiology, diagnosis and therapeutic management of sexual precocity". Arq Bras Endocrinol Metabol. 52 (1): 18–31. doi:10.1590/S0004-27302008000100005. PMID 18345393.
- ^ a b c d e Tindall DJ, Chang CH, Lobl TJ, Cunningham GR (1984). "Androgen antagonists in androgen target tissues". Pharmacol. Ther. 24 (3): 367–400. doi:10.1016/0163-7258(84)90010-x. PMID 6205409.
- ^ Namer M (October 1988). "Clinical applications of antiandrogens". J. Steroid Biochem. 31 (4B): 719–29. doi:10.1016/0022-4731(88)90023-4. PMID 2462132.
- ^ a b Fraser HM, Baird DT (February 1987). "Clinical applications of LHRH analogues". Baillière's Clin. Endocrinol. Metab. 1 (1): 43–70. doi:10.1016/S0950-351X(87)80052-6. PMID 3109366.
- ^ Laron Z, Kauli R (July 2000). "Experience with cyproterone acetate in the treatment of precocious puberty". J. Pediatr. Endocrinol. Metab. 13 (Suppl 1): 805–10. doi:10.1515/jpem.2000.13.s1.805. PMID 10969925. S2CID 25398066.
- ^ Neumann F, Kalmus J (1991). "Cyproterone acetate in the treatment of sexual disorders: pharmacological base and clinical experience". Exp. Clin. Endocrinol. 98 (2): 71–80. doi:10.1055/s-0029-1211103. PMID 1838080.
- ^ a b Holland FJ (March 1991). "Gonadotropin-independent precocious puberty". Endocrinol. Metab. Clin. North Am. 20 (1): 191–210. doi:10.1016/s0889-8529(18)30288-3. PMID 1903104.
- ^ a b Reiter EO, Norjavaara E (December 2005). "Testotoxicosis: current viewpoint". Pediatr Endocrinol Rev. 3 (2): 77–86. PMID 16361981.
- ^ Levey HR, Kutlu O, Bivalacqua TJ (2012). "Medical management of ischemic stuttering priapism: a contemporary review of the literature". Asian Journal of Andrology. 14 (1): 156–63. doi:10.1038/aja.2011.114. PMC 3753435. PMID 22057380.
- ^ Broderick GA, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R (2010). "Priapism: pathogenesis, epidemiology, and management". The Journal of Sexual Medicine. 7 (1 Pt 2): 476–500. doi:10.1111/j.1743-6109.2009.01625.x. PMID 20092449.
- ^ Chow K, Payne S (2008). "The pharmacological management of intermittent priapismic states". BJU International. 102 (11): 1515–21. doi:10.1111/j.1464-410X.2008.07951.x. PMID 18793304. S2CID 35399393.
- ^ Dahm P, Rao DS, Donatucci CF (2002). "Antiandrogens in the treatment of priapism". Urology. 59 (1): 138. doi:10.1016/S0090-4295(01)01492-3. PMID 11796309.
- ^ Yuan J, Desouza R, Westney OL, Wang R (2008). "Insights of priapism mechanism and rationale treatment for recurrent priapism". Asian Journal of Andrology. 10 (1): 88–101. doi:10.1111/j.1745-7262.2008.00314.x. PMID 18087648.
- ^ Baran R, Maibach HI (1 October 1998). Textbook of Cosmetic Dermatology. CRC Press. pp. 388–. ISBN 978-1-85317-478-0.
- ^ Maibach HI, Gorouhi F (2011). Evidence Based Dermatology. PMPH-USA. pp. 526–. ISBN 978-1-60795-039-4.
- ^ Williams H, Bigby M, Diepgen T, Herxheimer A, Naldi L, Rzany B (22 January 2009). Evidence-Based Dermatology. John Wiley & Sons. pp. 529–. ISBN 978-1-4443-0017-8. Archived from the original on 10 January 2023. Retrieved 27 December 2016.
- ^ a b Erem C (2013). "Update on idiopathic hirsutism: diagnosis and treatment". Acta Clin Belg. 68 (4): 268–74. doi:10.2143/ACB.3267. PMID 24455796. S2CID 39120534.
- ^ a b Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1004, 1196. ISBN 978-0-7817-1750-2.
- ^ a b c Camacho PM, Gharib H, Sizemore GW (2012). Evidence-Based Endocrinology. Lippincott Williams & Wilkins. pp. 199–. ISBN 978-1-4511-1091-3.
- ^ Randolph JF (December 2018). "Gender-Affirming Hormone Therapy for Transgender Females". Clin Obstet Gynecol. 61 (4): 705–721. doi:10.1097/GRF.0000000000000396. PMID 30256230. S2CID 52821192.
- ^ a b c d e f g h Singh SM, Gauthier S, Labrie F (February 2000). "Androgen receptor antagonists (antiandrogens): structure-activity relationships". Current Medicinal Chemistry. 7 (2): 211–247. doi:10.2174/0929867003375371. PMID 10637363.
- ^ a b c d Shen HC, Taplin ME, Balk SP (2010). "Androgen Receptor Antagonists". Drug Management of Prostate Cancer. Springer. pp. 71–81. doi:10.1007/978-1-60327-829-4_6. ISBN 978-1-60327-831-7.
- ^ Šauer P, Bořík A, Golovko O, Grabic R, Staňová AV, Valentová O, et al. (November 2018). "Do progestins contribute to (anti-)androgenic activities in aquatic environments?". Environmental Pollution. 242 (Pt A): 417–425. Bibcode:2018EPoll.242..417S. doi:10.1016/j.envpol.2018.06.104. PMID 29990947. S2CID 51622914.
- ^ Raudrant D, Rabe T (2003). "Progestogens with antiandrogenic properties". Drugs. 63 (5): 463–492. doi:10.2165/00003495-200363050-00003. PMID 12600226. S2CID 28436828.
- ^ Schneider HP (November 2003). "Androgens and antiandrogens". Annals of the New York Academy of Sciences. 997 (1): 292–306. Bibcode:2003NYASA.997..292S. doi:10.1196/annals.1290.033. PMID 14644837. S2CID 8400556.
- ^ a b c d e f Strauss III JF, Barbieri RL (13 September 2013). Yen and Jaffe's Reproductive Endocrinology. Elsevier Health Sciences. pp. 90–. ISBN 978-1-4557-2758-2.
- ^ Aggarwal S, Thareja S, Verma A, Bhardwaj TR, Kumar M (2010). "An overview on 5alpha-reductase inhibitors". Steroids. 75 (2): 109–53. doi:10.1016/j.steroids.2009.10.005. PMID 19879888. S2CID 44363501.
- ^ a b c Farmer PB, Walker JM (6 December 2012). The Molecular Basis of Cancer. Springer Science & Business Media. pp. 232–. ISBN 978-1-4684-7313-1.
- ^ a b c d e f Lemke TL, Williams DA (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 228–231, 1371–1372. ISBN 978-1-60913-345-0.
- ^ a b c de Lignières B, Silberstein S (April 2000). "Pharmacodynamics of oestrogens and progestogens". Cephalalgia: An International Journal of Headache. 20 (3): 200–7. doi:10.1046/j.1468-2982.2000.00042.x. PMID 10997774. S2CID 40392817.
- ^ Ledger W, Schlaff WD, Vancaillie TG (11 December 2014). Chronic Pelvic Pain. Cambridge University Press. pp. 55–. ISBN 978-1-316-21414-5.
- ^ a b Hanna L, Crosby T, Macbeth F (19 November 2015). Practical Clinical Oncology. Cambridge University Press. pp. 37–. ISBN 978-1-107-68362-4.
- ^ a b Weber GF (22 July 2015). Molecular Therapies of Cancer. Springer. pp. 314, 316. ISBN 978-3-319-13278-5.
- ^ Mahler C, Verhelst J, Denis L (May 1998). "Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer". Clin Pharmacokinet. 34 (5): 405–17. doi:10.2165/00003088-199834050-00005. PMID 9592622. S2CID 25200595.
- ^ a b Thomas JA (12 March 1997). Endocrine Toxicology, Second Edition. CRC Press. pp. 152–. ISBN 978-1-4398-1048-4. Archived from the original on 11 January 2023. Retrieved 27 December 2016.
- ^ Anderson J (2003). "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU Int. 91 (5): 455–61. doi:10.1046/j.1464-410x.2003.04026.x. PMID 12603397. S2CID 8639102.
- ^ Priestman T (26 May 2012). Cancer Chemotherapy in Clinical Practice. Springer Science & Business Media. pp. 97–. ISBN 978-0-85729-727-3.
- ^ a b c Higano CS (2003). "Side effects of androgen deprivation therapy: monitoring and minimizing toxicity". Urology. 61 (2 Suppl 1): 32–8. doi:10.1016/S0090-4295(02)02397-X. PMID 12667885.
- ^ Di Lorenzo G, Autorino R, Perdonà S, De Placido S (December 2005). "Management of gynaecomastia in patients with prostate cancer: a systematic review". Lancet Oncol. 6 (12): 972–9. doi:10.1016/S1470-2045(05)70464-2. PMID 16321765.
- ^ Shapiro J (12 November 2012). Hair Disorders: Current Concepts in Pathophysiology, Diagnosis and Management, An Issue of Dermatologic Clinics. Elsevier Health Sciences. pp. 187–. ISBN 978-1-4557-7169-1.
- ^ Futterweit W (6 December 2012). Polycystic Ovarian Disease. Springer Science & Business Media. pp. 282–. ISBN 978-1-4613-8289-8.
- ^ Katsambas AD, Dessinioti C (2010). "Hormonal therapy for acne: why not as first line therapy? facts and controversies". Clin. Dermatol. 28 (1): 17–23. doi:10.1016/j.clindermatol.2009.03.006. PMID 20082945.
- ^ Baldani DP, Skrgatic L, Ougouag R, Kasum M (February 2018). "The cardiometabolic effect of current management of polycystic ovary syndrome: strategies of prevention and treatment". Gynecol Endocrinol. 34 (2): 87–91. doi:10.1080/09513590.2017.1381681. PMID 28944709. S2CID 205631980.
- ^ Nakhjavani M, Hamidi S, Esteghamati A, Abbasi M, Nosratian-Jahromi S, Pasalar P (October 2009). "Short term effects of spironolactone on blood lipid profile: a 3-month study on a cohort of young women with hirsutism". Br J Clin Pharmacol. 68 (4): 634–7. doi:10.1111/j.1365-2125.2009.03483.x. PMC 2780289. PMID 19843067.
- ^ Cignarella A, Mioni R, Sabbadin C, Dassie F, Parolin M, Vettor R, Barbot M, Scaroni C (December 2020). "Pharmacological Approaches to Controlling Cardiometabolic Risk in Women with PCOS". Int J Mol Sci. 21 (24): 9554. doi:10.3390/ijms21249554. PMC 7765466. PMID 33334002.
- ^ Moretti C, Guccione L, Di Giacinto P, Simonelli I, Exacoustos C, Toscano V, Motta C, De Leo V, Petraglia F, Lenzi A (March 2018). "Combined Oral Contraception and Bicalutamide in Polycystic Ovary Syndrome and Severe Hirsutism: A Double-Blind Randomized Controlled Trial". J. Clin. Endocrinol. Metab. 103 (3): 824–838. doi:10.1210/jc.2017-01186. PMID 29211888.
- ^ Coleman, E.; et al. (19 August 2022). "Standards of Care for the Health of Transgender and Gender Diverse People, Version 8". International Journal of Transgender Health. 23 (Suppl 1): S1 – S259. doi:10.1080/26895269.2022.2100644. ISSN 2689-5269. PMC 9553112. PMID 36238954.
- ^ Godsland IF, Wynn V, Crook D, Miller NE (December 1987). "Sex, plasma lipoproteins, and atherosclerosis: prevailing assumptions and outstanding questions". American Heart Journal. 114 (6): 1467–1503. doi:10.1016/0002-8703(87)90552-7. PMID 3318361.
- ^ Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L, Virani SS, Yeboah J (June 2019). "2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines". Circulation. 139 (25): e1082 – e1143. doi:10.1161/CIR.0000000000000625. PMC 7403606. PMID 30586774.
- ^ a b Thole Z, Manso G, Salgueiro E, Revuelta P, Hidalgo A (2004). "Hepatotoxicity induced by antiandrogens: a review of the literature". Urol. Int. 73 (4): 289–95. doi:10.1159/000081585. PMID 15604569. S2CID 24799765.
- ^ Keating GM (March 2015). "Enzalutamide: a review of its use in chemotherapy-naïve metastatic castration-resistant prostate cancer". Drugs & Aging. 32 (3): 243–9. doi:10.1007/s40266-015-0248-y. PMID 25711765. S2CID 29563345.
- ^ a b Leppert PC, Peipert JF (2004). Primary Care for Women. Lippincott Williams & Wilkins. pp. 277–. ISBN 978-0-7817-3790-6.
- ^ Rathus SA, Nevid JS, Fichner-Rathus L (2005). Human sexuality in a world of diversity. Pearson Allyn and Bacon. p. 313. ISBN 978-0-205-40615-9. Archived from the original on 2023-02-26. Retrieved 2016-12-27.
- ^ Moguilewsky M, Bouton MM (October 1988). "How the study of the biological activities of antiandrogens can be oriented towards the clinic". Journal of Steroid Biochemistry. 31 (4B): 699–710. doi:10.1016/0022-4731(88)90021-0. PMID 3059062.
- ^ Gaillard-Moguilewsky M (1991). "Pharmacology of antiandrogens and value of combining androgen suppression with antiandrogen therapy". Urology. 37 (2 Suppl): 5–12. doi:10.1016/0090-4295(91)80095-O. PMID 1992602.
- ^ Moguilewsky M, Fiet J, Tournemine C, Raynaud JP (January 1986). "Pharmacology of an antiandrogen, anandron, used as an adjuvant therapy in the treatment of prostate cancer". Journal of Steroid Biochemistry. 24 (1): 139–46. doi:10.1016/0022-4731(86)90043-9. PMID 3009970.
- ^ Teutsch G, Goubet F, Battmann T, Bonfils A, Bouchoux F, Cerede E, Gofflo D, Gaillard-Kelly M, Philibert D (January 1994). "Non-steroidal antiandrogens: synthesis and biological profile of high-affinity ligands for the androgen receptor". The Journal of Steroid Biochemistry and Molecular Biology. 48 (1): 111–9. doi:10.1016/0960-0760(94)90257-7. PMID 8136296. S2CID 31404295.
- ^ Raynaud JP, Fortin M, Hunt P, Ojasoo T, Doré JC, Surcouf E, Mornon JP (1986). "Approaches to drug development using receptors". In Gotto AM, O'Malley BW, Liliane FP (eds.). The Role of Receptors in Biology and Medicine: Proceedings of the Ninth Argenteuil Symposium. Raven Press. pp. 65–77. ISBN 978-0-88167-161-2.
- ^ Raynaud JP, Ojasoo T, Labrie F (1981). "Steroid hormones—agonists and antagonists". Mechanisms of Steroid Action. Macmillan Education UK. pp. 145–158. doi:10.1007/978-1-349-81345-2_11. ISBN 978-1-349-81347-6.
- ^ Raynaud JP, Bouton MM, Moguilewsky M, Ojasoo T, Philibert D, Beck G, Labrie F, Mornon JP (January 1980). "Steroid hormone receptors and pharmacology". Journal of Steroid Biochemistry. 12: 143–57. doi:10.1016/0022-4731(80)90264-2. PMID 7421203.
- ^ Ojasoo T, Raynaud JP (November 1978). "Unique steroid congeners for receptor studies". Cancer Research. 38 (11 Pt 2): 4186–98. PMID 359134. Archived from the original on 2020-11-27. Retrieved 2021-10-31.
- ^ Raynaud JP, Bonne C, Bouton MM, Moguilewsky M, Philibert D, Azadian-Boulanger G (May 1975). "Screening for anti-hormones by receptor studies". Journal of Steroid Biochemistry. 6 (5): 615–22. doi:10.1016/0022-4731(75)90042-4. PMID 171505.
- ^ Hanada K, Furuya K, Yamamoto N, Nejishima H, Ichikawa K, Nakamura T, Miyakawa M, Amano S, Sumita Y, Oguro N (November 2003). "Bone anabolic effects of S-40503, a novel nonsteroidal selective androgen receptor modulator (SARM), in rat models of osteoporosis". Biol. Pharm. Bull. 26 (11): 1563–9. doi:10.1248/bpb.26.1563. PMID 14600402.
- ^ a b c d e Poyet P, Labrie F (October 1985). "Comparison of the antiandrogenic/androgenic activities of flutamide, cyproterone acetate and megestrol acetate". Molecular and Cellular Endocrinology. 42 (3): 283–8. doi:10.1016/0303-7207(85)90059-0. PMID 3930312. S2CID 24746807.
- ^ Luthy IA, Begin DJ, Labrie F (1988). "Androgenic activity of synthetic progestins and spironolactone in androgen-sensitive mouse mammary carcinoma (Shionogi) cells in culture". Journal of Steroid Biochemistry. 31 (5): 845–52. doi:10.1016/0022-4731(88)90295-6. PMID 2462135.
- ^ Sundar S, Dickinson PD (2012). "Spironolactone, a possible selective androgen receptor modulator, should be used with caution in patients with metastatic carcinoma of the prostate". BMJ Case Rep. 2012: bcr1120115238. doi:10.1136/bcr.11.2011.5238. PMC 3291010. PMID 22665559.
- ^ Flynn T, Guancial EA, Kilari M, Kilari D (2016). "Case Report: Spironolactone Withdrawal Associated With a Dramatic Response in a Patient With Metastatic Castrate-Resistant Prostate Cancer". Clin Genitourin Cancer. 15 (1): e95 – e97. doi:10.1016/j.clgc.2016.08.006. PMID 27641657. S2CID 38441469.
- ^ James VH, Pasqualini JR (22 October 2013). Hormonal Steroids: Proceedings of the Sixth International Congress on Hormonal Steroids. Elsevier Science. pp. 391–. ISBN 978-1-4831-9067-9.
- ^ a b Caubet JF, Tosteson TD, Dong EW, Naylon EM, Whiting GW, Ernstoff MS, Ross SD (1997). "Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens". Urology. 49 (1): 71–8. doi:10.1016/S0090-4295(96)00325-1. PMID 9000189.
Because steroidal antiandrogens such as cyproterone acetate have intrinsic androgenic activity and lower antiandrogenic activity than the NSAAs such as flutamide and nilutamide,39–43 it is not surprising that the two classes of antiandrogens may have different efficacies.
- ^ a b c Singh SM, Gauthier S, Labrie F (February 2000). "Androgen receptor antagonists (antiandrogens): structure-activity relationships". Current Medicinal Chemistry. 7 (2): 211–47. doi:10.2174/0929867003375371. PMID 10637363.
- ^ a b c d Ayub M, Levell MJ (August 1989). "The effect of ketoconazole related imidazole drugs and antiandrogens on [3H] R 1881 binding to the prostatic androgen receptor and [3H]5 alpha-dihydrotestosterone and [3H]cortisol binding to plasma proteins". J. Steroid Biochem. 33 (2): 251–5. doi:10.1016/0022-4731(89)90301-4. PMID 2788775.
- ^ a b Yamasaki K, Sawaki M, Noda S, Muroi T, Takakura S, Mitoma H, Sakamoto S, Nakai M, Yakabe Y (2004). "Comparison of the Hershberger assay and androgen receptor binding assay of twelve chemicals". Toxicology. 195 (2–3): 177–86. Bibcode:2004Toxgy.195..177Y. doi:10.1016/j.tox.2003.09.012. PMID 14751673.
- ^ Pratt WB (1994). The Anticancer Drugs. Oxford University Press. pp. 220–. ISBN 978-0-19-506739-2.
In patients receiving flutamide at the usual dosage of 250 mg every 8 hours, the minimal plasma concentration of hydroxyflutamide is about 5 uM, which is 5,000 times the plasma concentration of testosterone (1 nM) in patients treated with an LHRH agonist.127 As hydroxyflutamide is only one percent as potent as testosterone in competing for binding to the androgen receptor,126 a plasma level of 5 uM hydroxyflutamide is required to ensure effective competition.127 [...] Both cyproterone acetate and flutamide have been demonstrated to be effective therapy (roughly equivalent to an estrogen) when used alone in the treatment of carcinoma of the prostate.123
- ^ Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC (2010). "Molecular cell biology of androgen receptor signalling". Int. J. Biochem. Cell Biol. 42 (6): 813–27. doi:10.1016/j.biocel.2009.11.013. PMID 19931639.
- ^ Wang C, Liu Y, Cao JM (2014). "G protein-coupled receptors: extranuclear mediators for the non-genomic actions of steroids". Int J Mol Sci. 15 (9): 15412–25. doi:10.3390/ijms150915412. PMC 4200746. PMID 25257522.
- ^ Lang F, Alevizopoulos K, Stournaras C (2013). "Targeting membrane androgen receptors in tumors". Expert Opin. Ther. Targets. 17 (8): 951–63. doi:10.1517/14728222.2013.806491. PMID 23746222. S2CID 23918273.
- ^ a b Pescovitz OH, Eugster EA (2004). Pediatric Endocrinology: Mechanisms, Manifestations, and Management. Lippincott Williams & Wilkins. pp. 248–. ISBN 978-0-7817-4059-3.
- ^ a b Buonocore G, Bracci R, Weindling M (28 January 2012). Neonatology: A Practical Approach to Neonatal Diseases. Springer Science & Business Media. pp. 1012–. ISBN 978-88-470-1405-3.
- ^ Jordan-Young RM (7 January 2011). Brain Storm. Harvard University Press. pp. 82–. ISBN 978-0-674-05879-8.
- ^ Blakemore JE, Berenbaum SA, Liben LS (13 May 2013). Gender Development. Psychology Press. pp. 115–. ISBN 978-1-135-07932-1.
- ^ Maggi M (30 January 2012). Hormonal Therapy for Male Sexual Dysfunction. John Wiley & Sons. pp. 6–. ISBN 978-0-470-65760-7.
- ^ a b c d e Imamura Y, Sadar MD (August 2016). "Androgen receptor targeted therapies in castration-resistant prostate cancer: Bench to clinic". International Journal of Urology. 23 (8): 654–665. doi:10.1111/iju.13137. PMC 6680212. PMID 27302572.
- ^ De Mol E, Fenwick RB, Phang CT, Buzón V, Szulc E, de la Fuente A, et al. (September 2016). "EPI-001, A Compound Active against Castration-Resistant Prostate Cancer, Targets Transactivation Unit 5 of the Androgen Receptor". ACS Chemical Biology. 11 (9): 2499–2505. doi:10.1021/acschembio.6b00182. PMC 5027137. PMID 27356095.
- ^ Martinez-Ariza G, Hulme C (2015). "Recent advances in allosteric androgen receptor inhibitors for the potential treatment of castration-resistant prostate cancer". Pharmaceutical Patent Analyst. 4 (5): 387–402. doi:10.4155/ppa.15.20. PMID 26389532.
- ^ a b Lai AC, Crews CM (February 2017). "Induced protein degradation: an emerging drug discovery paradigm". Nature Reviews. Drug Discovery. 16 (2): 101–114. doi:10.1038/nrd.2016.211. PMC 5684876. PMID 27885283.
- ^ a b Lai KP, Huang CK, Chang YJ, Chung CY, Yamashita S, Li L, et al. (February 2013). "New therapeutic approach to suppress castration-resistant prostate cancer using ASC-J9 via targeting androgen receptor in selective prostate cells". The American Journal of Pathology. 182 (2): 460–473. doi:10.1016/j.ajpath.2012.10.029. PMC 3562731. PMID 23219429.
- ^ "ASCJ 9". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 2018-03-04. Retrieved 2017-12-24.
- ^ Witjes FJ, Debruyne FM, Fernandez del Moral P, Geboers AD (May 1989). "Ketoconazole high dose in management of hormonally pretreated patients with progressive metastatic prostate cancer. Dutch South-Eastern Urological Cooperative Group". Urology. 33 (5): 411–5. doi:10.1016/0090-4295(89)90037-X. PMID 2652864.
- ^ a b Held-Warmkessel J (2006). Contemporary Issues in Prostate Cancer: A Nursing Perspective. Jones & Bartlett Learning. pp. 275–. ISBN 978-0-7637-3075-8.
- ^ Bird IM, Abbott DH (2016). "The hunt for a selective 17,20 lyase inhibitor; learning lessons from nature". J. Steroid Biochem. Mol. Biol. 163: 136–46. doi:10.1016/j.jsbmb.2016.04.021. PMC 5046225. PMID 27154414.
- ^ Flores E, Bratoeff E, Cabeza M, Ramirez E, Quiroz A, Heuze I (May 2003). "Steroid 5alpha-reductase inhibitors". Mini-Reviews in Medicinal Chemistry. 3 (3): 225–37. doi:10.2174/1389557033488196. PMID 12570838.
- ^ Mozayani A, Raymon L (18 September 2011). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 656–. ISBN 978-1-61779-222-9.
- ^ a b c Bhagavan NV (2002). Medical Biochemistry. Academic Press. pp. 787–. ISBN 978-0-12-095440-7.
- ^ Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS (2016). "Adverse Effects and Safety of 5-alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review". J Clin Aesthet Dermatol. 9 (7): 56–62. PMC 5023004. PMID 27672412.
- ^ Stege R, Gunnarsson PO, Johansson CJ, Olsson P, Pousette A, Carlström K (1996). "Pharmacokinetics and testosterone suppression of a single dose of polyestradiol phosphate (Estradurin) in prostatic cancer patients". Prostate. 28 (5): 307–10. doi:10.1002/(SICI)1097-0045(199605)28:5<307::AID-PROS6>3.0.CO;2-8. PMID 8610057. S2CID 33548251.
- ^ Fourcade RO, McLeod D (2015). "Tolerability of Antiandrogens in the Treatment of Prostate Cancer". UroOncology. 4 (1): 5–13. doi:10.1080/1561095042000191655. ISSN 1561-0950.
- ^ Urotext (1 January 2001). Urotext-Luts: Urology. Urotext. pp. 71–. ISBN 978-1-903737-03-3. Archived from the original on 2023-01-11. Retrieved 2016-12-27.
- ^ Neumann F (1978). "The physiological action of progesterone and the pharmacological effects of progestogens--a short review". Postgraduate Medical Journal. 54 (Suppl 2): 11–24. PMID 368741.
- ^ Jacobi GH, Altwein JE, Kurth KH, Basting R, Hohenfellner R (1980). "Treatment of advanced prostatic cancer with parenteral cyproterone acetate: a phase III randomised trial". Br J Urol. 52 (3): 208–15. doi:10.1111/j.1464-410x.1980.tb02961.x. PMID 7000222.
- ^ Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2938–. ISBN 978-1-4160-6911-9. Archived from the original on 11 January 2023. Retrieved 27 December 2016.
- ^ Kjeld JM, Puah CM, Kaufman B, Loizou S, Vlotides J, Gwee HM, Kahn F, Sood R, Joplin GF (1979). "Effects of norgestrel and ethinyloestradiol ingestion on serum levels of sex hormones and gonadotrophins in men". Clinical Endocrinology. 11 (5): 497–504. doi:10.1111/j.1365-2265.1979.tb03102.x. PMID 519881. S2CID 5836155.
- ^ Norman G, Dean ME, Langley RE, Hodges ZC, Ritchie G, Parmar MK, Sydes MR, Abel P, Eastwood AJ (2008). "Parenteral oestrogen in the treatment of prostate cancer: a systematic review". Br. J. Cancer. 98 (4): 697–707. doi:10.1038/sj.bjc.6604230. PMC 2259178. PMID 18268497.
- ^ Dekanski JB (1980). "Anti-prostatic activity of bifluranol, a fluorinated bibenzyl". Br. J. Pharmacol. 71 (1): 11–6. doi:10.1111/j.1476-5381.1980.tb10903.x. PMC 2044395. PMID 6258683.
- ^ Sander S, Nissen-Meyer R, Aakvaag A (1978). "On gestagen treatment of advanced prostatic carcinoma". Scand. J. Urol. Nephrol. 12 (2): 119–21. doi:10.3109/00365597809179977. PMID 694436.
- ^ Prentky RA, Burgess AW (31 July 2000). Forensic Management of Sexual Offenders. Springer Science & Business Media. pp. 219–. ISBN 978-0-306-46278-8.
- ^ Sudo K, Yamazaki I, Masuoka M, Nakayama R (1979). "Anti-androgen TSAA-291. IV. Effects of the anti-androgen TSAA-291 (16 beta-ethyl-17 beta-hydroxy-4-oestren-3-one) on the secretion of gonadotrophins". Acta Endocrinol Suppl (Copenh). 229: 53–66. doi:10.1530/acta.0.092s053. PMID 294107.
- ^ a b Nieschlag E, Behre HM, Nieschlag S (26 July 2012). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 62–. ISBN 978-1-107-01290-5.
- ^ a b c IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 157–. ISBN 978-92-832-1291-1.
- ^ Coss CC, Jones A, Parke DN, Narayanan R, Barrett CM, Kearbey JD, Veverka KA, Miller DD, Morton RA, Steiner MS, Dalton JT (2012). "Preclinical characterization of a novel diphenyl benzamide selective ERα agonist for hormone therapy in prostate cancer". Endocrinology. 153 (3): 1070–81. doi:10.1210/en.2011-1608. PMID 22294742.
- ^ a b Krishna UR, Sheriar NK (1 January 2000). Adolescent Gynecology (pb). Orient Blackswan. pp. 121–. ISBN 978-81-250-1793-6.
- ^ Filshie M, Guillebaud J (22 October 2013). Contraception: Science and Practice. Elsevier Science. pp. 26–. ISBN 978-1-4831-6366-6.
- ^ a b Oettel M (1999). "Estrogens and Antiestrogens in the Male". Estrogens and Antiestrogens II. Handbook of Experimental Pharmacology. Vol. 135 / 2. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 505–571. doi:10.1007/978-3-642-60107-1_25. ISBN 978-3-642-64261-6. ISSN 0171-2004.
- ^ a b Margioris AN, Chrousos GP (20 April 2001). Adrenal Disorders. Springer Science & Business Media. pp. 84–. ISBN 978-1-59259-101-5.
- ^ a b c Polderman KH, Gooren LJ, van der Veen EA (October 1995). "Effects of gonadal androgens and oestrogens on adrenal androgen levels". Clin. Endocrinol. (Oxf). 43 (4): 415–21. doi:10.1111/j.1365-2265.1995.tb02611.x. PMID 7586614. S2CID 6815423.
- ^ Stege R, Eriksson A, Henriksson P, Carlström K (August 1987). "Orchidectomy or oestrogen treatment in prostatic cancer: effects on serum levels of adrenal androgens and related steroids". Int. J. Androl. 10 (4): 581–7. doi:10.1111/j.1365-2605.1987.tb00357.x. PMID 2958420.
- ^ a b c Pousette A, Carlström K, Stege R (1989). "Androgens during different modes of endocrine treatment of prostatic cancer". Urol. Res. 17 (2): 95–8. doi:10.1007/BF00262027. PMID 2734983. S2CID 25309877.
- ^ a b c Cox RL, Crawford ED (December 1995). "Estrogens in the treatment of prostate cancer". J. Urol. 154 (6): 1991–8. doi:10.1016/S0022-5347(01)66670-9. PMID 7500443.
- ^ von Schoultz B, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A, Stege R (1989). "Estrogen therapy and liver function--metabolic effects of oral and parenteral administration". Prostate. 14 (4): 389–95. doi:10.1002/pros.2990140410. PMID 2664738. S2CID 21510744.
- ^ a b Melmed S, Polonsky KS, Larsen PR, Kronenberg HM (12 May 2011). Williams Textbook of Endocrinology E-Book. Elsevier Health Sciences. pp. 753–. ISBN 978-1-4377-3600-7.
- ^ a b Kumar V, Abbas AK, Fausto N, Aster JC (10 June 2009). Robbins & Cotran Pathologic Basis of Disease E-Book. Elsevier Health Sciences. pp. 1154–. ISBN 978-1-4377-2015-0.
- ^ a b Nikolakis G, Kyrgidis A, Zouboulis CC (August 2019). "Is There a Role for Antiandrogen Therapy for Hidradenitis Suppurativa? A Systematic Review of Published Data". American Journal of Clinical Dermatology. 20 (4): 503–513. doi:10.1007/s40257-019-00442-w. PMID 31073704. S2CID 149443722.
- ^ Patel R, Shah G (September 2017). "Effect of metformin on clinical, metabolic and endocrine outcomes in women with polycystic ovary syndrome: a meta-analysis of randomized controlled trials". Current Medical Research and Opinion. 33 (9): 1545–1557. doi:10.1080/03007995.2017.1279597. PMID 28058854. S2CID 4391302.
- ^ Guan Y, Wang D, Bu H, Zhao T, Wang H (2020). "The Effect of Metformin on Polycystic Ovary Syndrome in Overweight Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials". International Journal of Endocrinology. 2020: 5150684. doi:10.1155/2020/5150684. PMC 7519180. PMID 33014044.
- ^ a b Sreenan JM, Diskin MG (6 December 2012). Embryonic Mortality in Farm Animals. Springer Science & Business Media. pp. 172–. ISBN 978-94-009-5038-2.
- ^ a b Jindal SK, Sharma MC (2010). Biotechnology in Animal Health and Production. New India Publishing. pp. 77–. ISBN 978-93-80235-35-6.
- ^ Fritz MA, Speroff L (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 750–751, 963. ISBN 978-1-4511-4847-3.
- ^ Kavoussi P, Costabile RA, Salonia A (17 October 2012). Clinical Urologic Endocrinology: Principles for Men's Health. Springer Science & Business Media. pp. 7–. ISBN 978-1-4471-4404-5.
- ^ Advances in Drug Research. Academic Press. 12 August 1997. pp. 34–. ISBN 978-0-08-052628-7.
- ^ Jugdutt BI (19 February 2014). Aging and Heart Failure: Mechanisms and Management. Springer Science & Business Media. pp. 175–. ISBN 978-1-4939-0268-2. Archived from the original on 11 January 2023. Retrieved 27 December 2016.
- ^ Wermuth CG (2 May 2011). The Practice of Murl=https://books.google.com/books?id=Qmt1_DQkCpEC&pg=PA34. Academic Press. pp. 34–. ISBN 978-0-08-056877-5.
- ^ Azziz R (8 November 2007). Androgen Excess Disorders in Women. Springer Science & Business Media. pp. 382–. ISBN 978-1-59745-179-6.
- ^ Runnebaum BC, Rabe T, Kiesel L (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 136–. ISBN 978-3-642-73790-9.
- ^ Orfanos CE, Montagna W, Stüttgen G (6 December 2012). Hair Research: Status and Future Aspects; Proceedings of the First International Congress on Hair Research, Hamburg, March 13th–16, 1979. Springer Science & Business Media. pp. 587–. ISBN 978-3-642-81650-5.
- ^ Marks L (2010). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 76–78. ISBN 978-0-300-16791-7.
- ^ Horsky J, Presl J (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 112–. ISBN 978-94-009-8195-9.
- ^ Vitamins and Hormones. Academic Press. 18 May 1976. pp. 682–. ISBN 978-0-08-086630-7.
- ^ Neal DE (6 December 2012). Tumours in Urology. Springer Science & Business Media. pp. 233–. ISBN 978-1-4471-2086-5.
- ^ Ottow E, Weinmann H (8 September 2008). Nuclear Receptors as Drug Targets. John Wiley & Sons. pp. 255–. ISBN 978-3-527-62330-3.
- ^ Singhal RL, Thomas JA (1 January 1976). Cellular Mechanisms Modulating Gonadal Action. University Park Press. p. 239. ISBN 978-0-8391-0776-7.
- ^ Liu B, Su L, Geng J, Liu J, Zhao G (October 2010). "Developments in nonsteroidal antiandrogens targeting the androgen receptor". ChemMedChem. 5 (10): 1651–1661. doi:10.1002/cmdc.201000259. PMID 20853390. S2CID 23228778.
- ^ Heyns W, Verhoeven G, De Moor P (May 1976). "Androgen binding in rat uterus cytosol. Study of the specificity". Journal of Steroid Biochemistry. 7 (5): 335–343. doi:10.1016/0022-4731(76)90092-3. PMID 180344.
- ^ Annual Reports in Medicinal Chemistry. Academic Press. 16 September 1986. pp. 182–. ISBN 978-0-08-058365-5.
- ^ Boris A, Scott JW, DeMartino L, Cox DC (March 1973). "Endocrine profile of a nonsteroidal antiandrogen N-(3,5-dimethyl-4-isoxazolylmethyl)phthalimide (DIMP)". Acta Endocrinologica. 72 (3): 604–614. doi:10.1530/acta.0.0720604. PMID 4739363.
- ^ a b c Bégué JP, Bonnet-Delpon D (2 June 2008). Bioorganic and Medicinal Chemistry of Fluorine. John Wiley & Sons. pp. 327–. ISBN 978-0-470-28187-1. Archived from the original on 12 January 2023. Retrieved 27 December 2016.
- ^ Menon MP, Higano CS (April 2013). "Enzalutamide, a second generation androgen receptor antagonist: development and clinical applications in prostate cancer". Current Oncology Reports. 15 (2): 69–75. doi:10.1007/s11912-013-0293-9. PMID 23341368. S2CID 8725297.
- ^ a b Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. (May 2009). "Development of a second-generation antiandrogen for treatment of advanced prostate cancer". Science. 324 (5928): 787–790. Bibcode:2009Sci...324..787T. doi:10.1126/science.1168175. PMC 2981508. PMID 19359544.
- ^ "FDA approves new treatment for a certain type of prostate cancer using novel clinical trial endpoint". Food and Drug Administration. 24 March 2020. Archived from the original on 23 April 2019. Retrieved 1 April 2018.
- ^ Sneader W (23 June 2005). Drug Discovery: A History. John Wiley & Sons. pp. 367–. ISBN 978-0-471-89979-2.
- ^ Golan DE (2008). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. pp. 624–. ISBN 978-0-7817-8355-2. Archived from the original on 2023-01-10. Retrieved 2016-12-27.
- ^ Prostate Cancer. Demos Medical Publishing. 20 December 2011. pp. 518–. ISBN 978-1-935281-91-7.
- ^ Bowsher W, Carter A (15 April 2008). Challenges in Prostate Cancer. John Wiley & Sons. pp. 138–. ISBN 978-1-4051-7177-9.
- ^ Allahbadia G, Agrawal R, Merchant R (2007). Polycystic Ovary Syndrome. Anshan. pp. 184–. ISBN 978-1-904798-74-3.
- ^ Frontiers in Medicinal Chemistry. Bentham Science Publishers. 2010. pp. 329–. ISBN 978-1-60805-208-0.
- ^ a b "Elagolix - Abbvie/Neurocrine Biosciences". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 2018-09-20. Retrieved 2018-07-30.
- ^ Crawford ED, Schellhammer PF, McLeod DG, Moul JW, Higano CS, Shore N, Denis L, Iversen P, Eisenberger MA, Labrie F (November 2018). "Androgen Receptor Targeted Treatments of Prostate Cancer: 35 Years of Progress with Antiandrogens". J. Urol. 200 (5): 956–966. doi:10.1016/j.juro.2018.04.083. PMID 29730201. S2CID 19162538.
- ^ a b c Kolkhof P, Bärfacker L (July 2017). "30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid receptor antagonists: 60 years of research and development". J. Endocrinol. 234 (1): T125 – T140. doi:10.1530/JOE-16-0600. PMC 5488394. PMID 28634268.
- ^ Pucci E, Petraglia F (December 1997). "Treatment of androgen excess in females: yesterday, today and tomorrow". Gynecol. Endocrinol. 11 (6): 411–33. doi:10.3109/09513599709152569. PMID 9476091.
- ^ Smith WG (1962). "Spironolactone and gynaecomastia". The Lancet. 280 (7261): 886. doi:10.1016/S0140-6736(62)90668-2. ISSN 0140-6736.
- ^ a b Stewart ME, Pochi PE (April 1978). "Antiandrogens and the skin". Int. J. Dermatol. 17 (3): 167–79. doi:10.1111/j.1365-4362.1978.tb06057.x. PMID 148431. S2CID 43649686.
- ^ Zarate A, Mahesh VB, Greenblatt RB (December 1966). "Effect of an antiandrogen, 17-alpha-methyl-B-nortestosterone, on acne and hirsutism". J. Clin. Endocrinol. Metab. 26 (12): 1394–8. doi:10.1210/jcem-26-12-1394. PMID 4225258.
- ^ Hamada H, Neumann F, Junkmann K (November 1963). "Intrauterine Antimaskuline Beeinflussung von Rattenfeten Durch ein Stark Gestagen Wirksames Steroid" [Intrauterine antimasculine influence of Rat Fetuses by Birtue of a Powerful Steroid Acting as a Progestogen]. Acta Endocrinologica (in German). 44 (3): 380–388. doi:10.1530/acta.0.0440380. PMID 14071315.
- ^ Thibaut F, De La Barra F, Gordon H, Cosyns P, Bradford JM (June 2010). "The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of paraphilias". World J. Biol. Psychiatry. 11 (4): 604–55. doi:10.3109/15622971003671628. PMID 20459370. S2CID 14949511.
- ^ Hammerstein J, Cupceancu B (April 1969). "Behandlung des Hirsutismus mit Cyproteronacetat" [Management of hirsutism using cyproterone acetate]. Deutsche Medizinische Wochenschrift (in German). 94 (16): 829–34. doi:10.1055/s-0028-1111126. ISSN 0012-0472. PMID 4304873. S2CID 71214286.
- ^ Steelman SL, Brooks JR, Morgan ER, Patanelli DJ (1969). "Anti-androgenic activity of spironolactone". Steroids. 14 (4): 449–50. doi:10.1016/s0039-128x(69)80007-3. PMID 5344274.
- ^ Neri R, Florance K, Koziol P, Van Cleave S (August 1972). "A biological profile of a nonsteroidal antiandrogen, SCH 13521 (4'-nitro-3'trifluoromethylisobutyranilide)". Endocrinology. 91 (2): 427–37. doi:10.1210/endo-91-2-427. PMID 4264731.
- ^ Neri RO, Monahan M (September 1972). "Effects of a novel nonsteroidal antiandrogen on canine prostatic hyperplasia". Invest Urol. 10 (2): 123–30. PMID 4116667.
- ^ Neumann F (1994). "The antiandrogen cyproterone acetate: discovery, chemistry, basic pharmacology, clinical use and tool in basic research". Exp. Clin. Endocrinol. 102 (1): 1–32. doi:10.1055/s-0029-1211261. PMID 8005205.
- ^ Raynaud JP, Bonne C, Bouton MM, Lagace L, Labrie F (1979). "Action of a non-steroid anti-androgen, RU 23908, in peripheral and central tissues". J. Steroid Biochem. 11 (1A): 93–9. doi:10.1016/0022-4731(79)90281-4. PMID 385986.
- ^ Ober KP, Hennessy JF (1978). "Spironolactone therapy for hirsutism in a hyperandrogenic woman". Ann. Intern. Med. 89 (5 Pt 1): 643–4. doi:10.7326/0003-4819-89-5-643. PMID 717935.
- ^ Klotz L (May 2006). "Combined androgen blockade: an update". Urol. Clin. North Am. 33 (2): 161–6, v–vi. doi:10.1016/j.ucl.2005.12.001. PMID 16631454.
- ^ Labrie F, Dupont A, Belanger A, Cusan L, Lacourciere Y, Monfette G, Laberge JG, Emond JP, Fazekas AT, Raynaud JP, Husson JM (1982). "New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an antiandrogen". Clin Invest Med. 5 (4): 267–75. PMID 6819101.
- ^ Engel J, Kleemann A, Kutscher B, Reichert D (2009). Pharmaceutical Substances: Syntheses, Patents and Applications of the most relevant APIs (5th ed.). Thieme. pp. 153–154. ISBN 978-3-13-179275-4.
- ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1695–. ISBN 978-0-8155-1856-3. Archived from the original on 12 January 2023. Retrieved 2 January 2019.
- ^ The Irish Reports: Containing Reports of Cases Argued and Determined in the Court of Appeal, the High Court of Justice, the Court of Bankruptcy, in Ireland, and the Irish Land Commission. Incorporated Council of Law Reporting for Ireland. 1990. pp. 501–502. Archived from the original on 2023-01-12. Retrieved 2019-01-02.
- ^ Regitz-Zagrosek V (2 October 2012). Sex and Gender Differences in Pharmacology. Springer Science & Business Media. pp. 575–. ISBN 978-3-642-30725-6.
- ^ "Abiraterone: A story of scientific innovation and commercial partnership - the Institute of Cancer Research, London". Archived from the original on 2019-01-01. Retrieved 2019-01-02.
- ^ "Nilutamide". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 2021-05-05. Retrieved 2019-01-02.
- ^ Sawyers, C., Jung, M., Chen, C., Ouk, S., Welsbie, D., Tran, C., ... & Yoo, D. (2006). U.S. Patent Application No. 11/433,829. https://patents.google.com/patent/US20070004753 [1]
- ^ "Espacenet - Original document". Archived from the original on 2021-11-04. Retrieved 2019-01-02.
- ^ "Abiraterone acetate - Johnson & Johnson". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 2019-01-03. Retrieved 2019-01-02.
- ^ "Enzalutamide - Astellas Pharma/Medivation". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 2018-07-17. Retrieved 2019-01-02.
- ^ "Apalutamide - Janssen Research and Development". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 2019-01-03. Retrieved 2019-01-02.
- ^ "Relugolix - Myovant/Takeda". AdisInsight. Springer Nature Switzerland AG. Archived from the original on 2021-08-19. Retrieved 2021-08-19.
- ^ a b Neumann F (January 1977). "Pharmacology and potential use of cyproterone acetate". Hormone and Metabolic Research. 9 (1): 1–13. doi:10.1055/s-0028-1093574. PMID 66176. S2CID 7224893.
- ^ a b Dorfman RI (1970). "Biological Activity of Antiandrogens". British Journal of Dermatology. 82 (s6): 3. doi:10.1111/j.1365-2133.1970.tb07998.x. ISSN 0007-0963. S2CID 71393789.
- ^ a b Gräf KJ, Brotherton J, Neumann F (1974). "Clinical Uses of Antiandrogens". Androgens II and Antiandrogens / Androgene II und Antiandrogene. Springer. pp. 485–542. doi:10.1007/978-3-642-80859-3_7. ISBN 978-3-642-80861-6.
- ^ Vogelzang N (2006). Comprehensive Textbook of Genitourinary Oncology. Lippincott Williams & Wilkins. pp. 316–. ISBN 978-0-7817-4984-8.
- ^ a b c Helms RA, Quan DJ (2006). Textbook of Therapeutics: Drug and Disease Management. Lippincott Williams & Wilkins. pp. 211–. ISBN 978-0-7817-5734-8.
- ^ Farid NR, Diamanti-Kandarakis E (27 February 2009). Diagnosis and Management of Polycystic Ovary Syndrome. Springer Science & Business Media. pp. 235–. ISBN 978-0-387-09718-3. Archived from the original on 11 January 2023. Retrieved 30 December 2016.
- ^ Neumann F, Diallo FA, Hasan SH, Schenck B, Traore I (1976). "The influence of pharmaceutical compounds on male fertility". Andrologia. 8 (3): 203–35. doi:10.1111/j.1439-0272.1976.tb02137.x. PMID 793446. S2CID 24859886.
- ^ Prasad MR, Rajalakshmi M (1976). "Target sites for suppressing fertility in the male". Adv Sex Horm Res. 2: 263–87. PMID 797248.
- ^ Ewing LL, Robaire B (1978). "Endogenous antispermatogenic agents: prospects for male contraception". Annu. Rev. Pharmacol. Toxicol. 18: 167–87. doi:10.1146/annurev.pa.18.040178.001123. PMID 206192.
- ^ Gombe S (April 1983). "A review of the current status in male contraceptive studies". East Afr Med J. 60 (4): 203–11. PMID 6354690.
- ^ a b Srivastava RP, Bhaduri AP (1989). "Emerging concepts towards the development of contraceptive agents". In Ernst Jucker (ed.). Progress in Drug Research. Progress in Drug Research. Fortschritte der Arzneimittelforschung. Progres des Recherches Pharmaceutiques. Vol. 33. Birkhäuser. pp. 267–315. doi:10.1007/978-3-0348-9146-2_9. ISBN 978-3-0348-9925-3. PMID 2687939.
- ^ a b c Wu FC (October 1988). "Male contraception: current status and future prospects". Clin. Endocrinol. (Oxf). 29 (4): 443–65. doi:10.1111/j.1365-2265.1988.tb02894.x. PMID 3075164. S2CID 36608203.
- ^ a b Nieschlag E, Zitzmann M, Kamischke A (November 2003). "Use of progestins in male contraception". Steroids. 68 (10–13): 965–72. doi:10.1016/s0039-128x(03)00135-1. PMID 14667989. S2CID 22458746.
- ^ a b Nieschlag E (2010). "Clinical trials in male hormonal contraception" (PDF). Contraception. 82 (5): 457–70. doi:10.1016/j.contraception.2010.03.020. PMID 20933120. Archived (PDF) from the original on 2020-12-05. Retrieved 2019-07-08.
- ^ Rajalakshmi M (November 2005). "Male contraception: expanding reproductive choice". Indian J. Exp. Biol. 43 (11): 1032–41. PMID 16313066.
- ^ Chao JH, Page ST (July 2016). "The current state of male hormonal contraception". Pharmacol. Ther. 163: 109–17. doi:10.1016/j.pharmthera.2016.03.012. PMID 27016468.
- ^ Attardi BJ, Hild SA, Reel JR (June 2006). "Dimethandrolone undecanoate: a new potent orally active androgen with progestational activity". Endocrinology. 147 (6): 3016–26. doi:10.1210/en.2005-1524. PMID 16497801.
- ^ Ayoub R, Page ST, Swerdloff RS, Liu PY, Amory JK, Leung A, Hull L, Blithe D, Christy A, Chao JH, Bremner WJ, Wang C (March 2017). "Comparison of the single dose pharmacokinetics, pharmacodynamics, and safety of two novel oral formulations of dimethandrolone undecanoate (DMAU): a potential oral, male contraceptive". Andrology. 5 (2): 278–285. doi:10.1111/andr.12303. PMC 5352517. PMID 27907978.
- ^ Fioretti FM, Sita-Lumsden A, Bevan CL, Brooke GN (June 2014). "Revising the role of the androgen receptor in breast cancer". J. Mol. Endocrinol. 52 (3): R257–65. doi:10.1530/JME-14-0030. PMID 24740738.
- ^ Gucalp A, Traina TA (2016). "Targeting the androgen receptor in triple-negative breast cancer". Curr Probl Cancer. 40 (2–4): 141–150. doi:10.1016/j.currproblcancer.2016.09.004. PMC 5580391. PMID 27816190.
- ^ Arce-Salinas C, Riesco-Martinez MC, Hanna W, Bedard P, Warner E (February 2016). "Complete Response of Metastatic Androgen Receptor-Positive Breast Cancer to Bicalutamide: Case Report and Review of the Literature". J. Clin. Oncol. 34 (4): e21–4. doi:10.1200/JCO.2013.49.8899. PMID 24888812.
- ^ Héquet D, Mzoughi S, Rouzier R, Guccione E (April 2017). "[Androgen receptors in breast cancer: Expression, value and therapeutic prospects]". Bull Cancer (in French). 104 (4): 363–369. doi:10.1016/j.bulcan.2017.01.005. PMID 28216075.
- ^ Gerratana L, Basile D, Buono G, De Placido S, Giuliano M, Minichillo S, Coinu A, Martorana F, De Santo I, Del Mastro L, De Laurentiis M, Puglisi F, Arpino G (June 2018). "Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype". Cancer Treat. Rev. 68: 102–110. doi:10.1016/j.ctrv.2018.06.005. hdl:11567/914067. PMID 29940524.
- ^ Nomani H, Mohammadpour AH, Moallem SM, YazdanAbad MJ, Barreto GE, Sahebkar A (December 2019). "Anti-androgen drugs in the treatment of obsessive-compulsive disorder: a systematic review". Curr Med Chem. 27 (40): 6825–6836. doi:10.2174/0929867326666191209142209. PMID 31814547. S2CID 208956450.
Further reading
[edit]- Neumann F, Steinbeck H (1974). "Antiandrogens". Androgens II and Antiandrogens / Androgene II und Antiandrogene. Springer. pp. 235–484. doi:10.1007/978-3-642-80859-3_6. ISBN 978-3-642-80861-6.
- Gräf KJ, Brotherton J, Neumann F (1974). "Clinical Uses of Antiandrogens". Androgens II and Antiandrogens / Androgene II und Antiandrogene. Springer. pp. 485–542. doi:10.1007/978-3-642-80859-3_7. ISBN 978-3-642-80861-6.
