Ancylostoma duodenale
| Ancylostoma duodenale | |
|---|---|

| |
| Photomicrograph of larva | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Nematoda |
| Class: | Chromadorea |
| Order: | Rhabditida |
| Family: | Ancylostomatidae |
| Genus: | Ancylostoma |
| Species: | A. duodenale
|
| Binomial name | |
| Ancylostoma duodenale (Dubini, 1843)
| |
| Synonyms[1] | |
|
Agchylostoma duodenale Dubini, 1843 | |
Ancylostoma duodenale is a species of the roundworm genus Ancylostoma. It is a parasitic nematode worm and commonly known as the Old World hookworm. It lives in the small intestine especially the jejunum[citation needed] of definitive hosts, generally humans,[2]: 307–308 [3] where it is able to mate and mature. Ancylostoma duodenale and Necator americanus are the two human hookworm species that are normally discussed together as the cause of hookworm infection. They are dioecious.[4] Ancylostoma duodenale is abundant throughout the world, including Southern Europe, North Africa, India, China, Southeast Asia, some areas in the United States, the Caribbean, and South America.
Characteristics
[edit]A. duodenale is small, cylindrical worm, greyish-white in color. It has two ventral plates on the anterior margin of the buccal capsule. Each of them has two large teeth that are fused at their bases. A pair of small teeth can be found in the depths of the buccal capsule. Males are 8–11 mm long with a copulatory bursa at the posterior end. Females are 10–13 mm long, with the vulva located at the posterior end; females can lay 10,000 to 30,000 eggs per day. The average lifespan of a female A. duodenale is one year.[5] There are four larval stages, and the filariform larvae (L3) are sheathed because they retain the cuticle of the prior developmental stage after molting.[2]: 307–308
Lifecycle
[edit]After a filariform "infective" larva penetrates the intact skin – most commonly through the feet – the larva enters the blood circulation. It is then carried to the lungs, breaks into alveoli, ascends the bronchi and trachea, and is coughed up and swallowed back into the small intestine, where it matures. The larva later matures into an adult in the small intestine (jejunum mainly[citation needed]), where they attach to the villi and female worms can lay 25,000 eggs per day. The eggs are released into the feces and reside on soil; when deposited on warm, moist soil, a larva rapidly develops in the egg and hatches after one to two days. This rhabditiform larva moults twice in the soil and becomes a skin-penetrating third-stage infective larva within 5 to 10 days. These infective filariform larvae are able to sense vibrations in the soil, heat, or carbon dioxide, and employ dendritic processes similar to cilia. They use these processes as thermosensory, chemosensory, and mechanosensory receptors to migrate towards a host for infection.[5] The filariform larvae (L3 stage) can then penetrate the exposed skin of another organism and begin a new cycle of infection. A. duodenale can also be transmitted orally, mediated by ingestion of the filariform larvae;[2]: 307–308 it may have paratenic hosts in other mammals, in whom the larvae may survive in muscles.[6] Vertical transmission in people by breastfeeding[3][7] and by a transplacental route[6][7] has been reported.
Epidemiology
[edit]A. duodenale is prevalent in Southern Europe, North Africa, India, China, Southeast Asia, small areas of United States, the Caribbean islands, and South America. This hookworm is well known in mines because of the consistency in temperature and humidity that provides an ideal habitat for egg and juvenile development. An estimated one billion people are infected with hookworms. Transmission of A. duodenale is by contact of skin with soil contaminated with larvae. The way it enters the human body was understood in the 1880s, after an epidemic of ancylostomiasis among miners working in the hot and humid Gotthard Tunnel (Switzerland).[8][9]
Infection
[edit]A light hookworm infection causes abdominal pain, loss of appetite, and geophagy. Heavy infection causes severe protein deficiency or iron-deficiency anemia. Protein deficiency may lead to dry skin, edema, and abdominal distension from edema (potbelly), while iron-deficiency anemia might result in mental dullness and heart failure. In pregnant women, this parasite is able to infect the fetus and can cause complications such as low birth weight, maternal anemia, and infant mortality.[10]
The eggs of A. duodenale and Necator americanus cannot be distinguished. Larvae cannot be found in stool specimens unless they are left at ambient temperature for a day or more. Education, improved sanitation, and controlled disposal of human feces are important. Wearing shoes in endemic areas can reduce the prevalence of infection, as well. A. duodenale can be treated with albendazole, mebendazole, and benzimidazoles. Pyrantel pamoate is an alternative. In severe cases of anemia, blood transfusion may be necessary.[citation needed]
Gallery
[edit]-
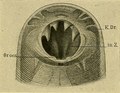
Buccal capsule of adult showing four teeth (arrows) on its ventral wall[2]: 307
-
Diagram of adult's head
-
Schematic of thin-shelled egg at eight-cell stage
-
Infective filariform larva
References
[edit]- ^ "Ancylostoma duodenale (Dubini, 1843) Creplin, 1845". Global Biodiversity Information Facility. Retrieved 9 September 2024.
- ^ a b c d Bogitsh BJ, Carter CE, Oeltmann TN (2013). "Chapter 16: Intestinal Nematodes". Human Parasitology (Fourth ed.). Elsevier. pp. 291–327. doi:10.1016/B978-0-12-415915-0.00016-9. ISBN 978-0-12-415915-0.
- ^ a b Kimberlin, David W.; Banerjee, Ritu; Barnett, Elizabeth D.; Lynfield, Ruth; Sawyer, Mark H. (2024). "Hookworm Infections (Necator americanus and Ancylostoma species)". Red Book: 2024–2027 Report of the Committee on Infectious Diseases (Report). doi:10.1542/9781610027373-S3_008_014.
- ^ Ferris, Howard (23 May 2005). "Ancylostoma duodenale". Nemaplex: Nematode-Plant Expert Information System. University of California, Davis. Archived from the original on 1 March 2009. Retrieved 22 January 2009.
- ^ a b Fetouh, Nagla (2003). Mulcrone, Renee Sherman; Friedrich, Teresa (eds.). "Ancylostoma duodenale". Animal Diversity Web. Retrieved 9 September 2024.
- ^ a b "Ancylostoma/Necator". The Australian Society for Parasitology Inc. Retrieved 4 December 2024.
- ^ a b Umbrello, G.; Pinzani, R.; Bandera, A.; Formenti, F.; Zavarise, G.; Arghittu, M.; Girelli, D.; Maraschini, A.; Muscatello, A.; Maraschisio, P.; Bosis, S. (2021). "Hookworm infection in infants: a case report and review of literature". Italian Journal of Pediatrics. 47 (26). doi:10.1186/s13052-021-00981-1. PMC 7871578. PMID 33563313.
- ^ Bugnion, E. (1881). "On the epidemic caused by Ankylostomum among the eorkmen in the St. Gothard Tunnel". British Medical Journal. 1 (1054): 382. doi:10.1136/bmj.1.1054.382. PMC 2263460. PMID 20749811.
- ^ Peduzzi, R.; Piffaretti, J.-C. (1983). "Ancylostoma duodenale and the Saint Gothard anaemia". British Medical Journal. 287 (6409): 1942–5. doi:10.1136/bmj.287.6409.1942. PMC 1550193. PMID 6418279.
- ^ "Soil-Transmitted Helminths | USAID's Neglected Tropical Disease Program". www.neglecteddiseases.gov. Archived from the original on 2017-04-26. Retrieved 2017-04-26.
Further reading
[edit]- Hotez, P.J.; Pritchard, D.I. (June 1995). "Hookworm infection". Sci. Am. 272 (6): 68–74. Bibcode:1995SciAm.272f..68H. doi:10.1038/scientificamerican0695-68. PMID 7761817.
- Looss, A. (1898). "Zur Lebensgeschichte des Ankylostoma duodenale". CBT. Bakt. 24: 441–9, 483–8.
- Murray, P.R.; Rosenthal, K.S.; Pfaller, M.A. (2009). Medical Microbiology (6th ed.). Elsevier/Mosby. ISBN 978-0323076791.
- Schmidt, G.D.; Roberts, L.S. (2009). Foundations of parasitology (8th ed.). McGraw-Hill. pp. 472–3. ISBN 978-0071311038.