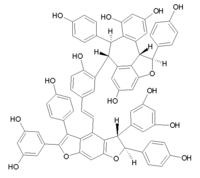
Amurensin E
Appearance
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(21S,26R,27S,211bS,4E,65S,66S)-65-(3,5-Dihydroxyphenyl)-27,63,66-tris(4-hydroxyphenyl)-21,26,27,211b,65,66-hexahydro-2(1,6)-benzo[6,7]cyclohepta[1,2,3-cd][1]benzofurana-6(4,2)-benzo[1,2-b:5,4-b′]difurana-1,7(1),3(1,3)-tribenzenaheptaphan-4-ene-14,24,28,210,36,73,75-heptol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C70H50O15 | |
| Molar mass | 1131.14 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Amurensin E is an oligostilbene found in Vitis amurensis.[1] It is a pentamer of resveratrol.
References
[edit]- ^ Four Novel Oligostilbenes from the Roots of Vitis amurensis. Kai-Sheng Huang, Mao Lin, Lin-Ning Yu and Man Kong, Tetrahedron, 3 March 2000, Volume 56, Issue 10, Pages 1321–1329, doi:10.1016/S0040-4020(99)01034-0
