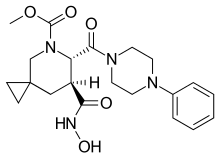
Aderbasib
Appearance
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID |
|
| ChemSpider | |
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H28N4O5 |
| Molar mass | 416.478 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Aderbasib (codenamed INCB7839) is a sheddase inhibitor that may suppress tumor cell proliferation.[1] Acting on multiple receptor classes and subclasses, aderbasib is observed to regulate the tumor necrosis factor of cancer cells.[2] Aderbasib was being developed by Incyte as a potential adjunctive treatment for metastatic breast cancer. Development was halted in 2011 after positive findings from Phase II trials were contradicted by further research.[3][4]
More specifically, aderbasib is an inhibitor of ADAM10 and ADAM17.[5]
References
[edit]- ^ "STATEMENT ON A NONPROPRIETARY NAME ADOPTED BY THE USAN COUNCIL" (PDF). Ama-assn.org. Retrieved 2012-10-13.
- ^ "Aderbasib | CAS#791828-58-5". MedKoo. Retrieved 2012-10-13.
- ^ Incyte (2011-09-19). "UBS 2011 Global Life Sciences Conference". Retrieved 2013-02-13.
- ^ "Incyte Reports Third Quarter 2011 Financial Results And Provides Update On Key Clinical Programs" (Press release). BusinessWire. 2011-10-27. Retrieved 2013-02-13.
- ^ Yang L, Bhattacharya A, Li Y, Sexton S, Ling X, Li F, et al. (June 2022). "Depleting receptor tyrosine kinases EGFR and HER2 overcomes resistance to EGFR inhibitors in colorectal cancer". Journal of Experimental & Clinical Cancer Research. 41 (1): 184. doi:10.1186/s13046-022-02389-z. PMC 9161494. PMID 35650607.
