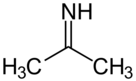
Acetone imine
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propan-2-imine[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| MeSH | Imine Acetone Imine | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H7N | |||
| Molar mass | 57.096 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Density | 0.8 g cm−3 (25 °C) | ||
| Boiling point | 57–59 °C (135–138 °F; 330–332 K) | ||
| log P | -0.56 | ||
Refractive index (nD)
|
1.394 | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H225, H319, H336 | |||
| P210, P261, P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 14.7 °C (58.5 °F; 287.8 K) | ||
| Related compounds | |||
Related compounds
|
Acetone oxime | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Acetone imine, or 2-propanimine is an organic compound and an imine with the chemical formula (CH3)2CNH. It is a volatile and flammable liquid at room temperature. It is the simplest ketimine. This compound is mainly of academic interest.
Synthesis and reactions
[edit]Acetone imine is prepared by dehydrocyanation of the cyanoamine of acetone, which is prepared from acetone cyanohydrin. Dicyclohexylcarbodiimide (CyN=C=NCy) serves as the scavenger for hydrogen cyanide:[2]
- (CH3)2C(NH2)CN + CyN=C=NCy → (CH3)2CNH + CyN(H)-C(CN)=NCy

Upon standing at room temperature, samples of acetone imine degrade to give this heterocycle, called acetonin.
The compound hydrolyzes readily:
- (CH3)2CNH + H2O → (CH3)2CO + NH3
This reactivity is characteristic of imines derived from ammonia. Methylene imine (CH2=NH) is also highly reactive, condensing to hexamethylenetetramine. Upon standing, acetone imine undergoes further condensation to give the tetrahydropyrimidine called acetonin, with loss of ammonia.[3]
The imine of hexafluoroacetone, ((CF3)2C=NH) is by contrast robust.[4]
References
[edit]- ^ "Synonyms". Pubchem.
- ^ K. Findeisen; H. Heitzer; K. Dehnicke (1981). "Neue Methode zur Herstellung von Aldiminen und Ketiminen". Synthesis. 1981 (9): 702–704. doi:10.1055/s-1981-29566. S2CID 98811861.
- ^ Matter, E. (1947). "Über ein neues Reaktionsprodukt aus Aceton und Ammoniak (Acetonin) (A new reaction product from acetone and ammonia (acetonine)) I". Helvetica Chimica Acta. 30: 1114–23. doi:10.1002/hlca.19470300503.
- ^ W. J. Middleton, H. D. Carlson (1970). "Hexafluoroacetone Imine". Org. Syntheses. 50: 81–3. doi:10.15227/orgsyn.050.0081..