Paracetamol poisoning
| Paracetamol poisoning | |
|---|---|
| Other names | Acetaminophen toxicity, paracetamol toxicity, acetaminophen poisoning, paracetamol overdose, acetaminophen overdose, Tylenol toxicity |
 | |
| Paracetamol | |
| Specialty | Toxicology |
| Symptoms | Early: Non specific, feeling tired, abdominal pain, nausea Later: Yellowish skin, blood clotting problems, confusion |
| Complications | Liver failure, kidney failure, pancreatitis, low blood sugar, lactic acidosis. |
| Usual onset | After 24 hours (toxicity)[1] |
| Causes | Paracetamol (acetaminophen) usually > 7 g[2][1] |
| Risk factors | Alcoholism, malnutrition, certain other hepatotoxic medications[1] |
| Diagnostic method | Blood levels at specific times following use[1] |
| Differential diagnosis | Alcoholism, viral hepatitis, gastroenteritis[1] |
| Treatment | Activated charcoal, acetylcysteine, liver transplant[1] |
| Prognosis | Death occurs in ~0.1%[1] |
| Frequency | >100,000 per year (US)[1] |
Paracetamol poisoning, also known as acetaminophen poisoning, is caused by excessive use of the medication paracetamol (acetaminophen).[2] Most people have few or non-specific symptoms in the first 24 hours following overdose. These symptoms include feeling tired, abdominal pain, or nausea. This is typically followed by absence of symptoms for a couple of days, after which yellowish skin, blood clotting problems, and confusion occurs as a result of liver failure. Additional complications may include kidney failure, pancreatitis, low blood sugar, and lactic acidosis. If death does not occur, people tend to recover fully over a couple of weeks.[3][4] Without treatment, death from toxicity occurs 4 to 18 days later.[5]
Paracetamol poisoning can occur accidentally or as an attempt to die by suicide. Risk factors for toxicity include alcoholism, malnutrition, and the taking of certain other hepatotoxic medications.[1] Liver damage results not from paracetamol itself, but from one of its metabolites, N-acetyl-p-benzoquinone imine (NAPQI).[6] NAPQI decreases the liver's glutathione and directly damages cells in the liver.[7] Diagnosis is based on the blood level of paracetamol at specific times after the medication was taken.[1] These values are often plotted on the Rumack-Matthew nomogram to determine level of concern.[1]
Treatment may include activated charcoal if the person seeks medical help soon after the overdose.[1] Attempting to force the person to vomit is not recommended.[6] If there is a potential for toxicity, the antidote acetylcysteine is recommended.[1] The medication is generally given for at least 24 hours.[6] Psychiatric care may be required following recovery.[1] A liver transplant may be required if damage to the liver becomes severe. The need for transplant is often based on low blood pH, high blood lactate, poor blood clotting, or significant hepatic encephalopathy. With early treatment liver failure is rare.[6] Death occurs in about 0.1% of cases.[1]
Paracetamol poisoning was first described in the 1960s.[6] Rates of poisoning vary significantly between regions of the world.[8] In the United States more than 100,000 cases occur a year.[1] In the United Kingdom it is the medication responsible for the greatest number of overdoses.[7] Young children are most commonly affected.[1] In the United States and the United Kingdom, paracetamol is the most common cause of acute liver failure.[9][1]
Signs and symptoms
[edit]The signs and symptoms of paracetamol toxicity occur in three phases. The first phase begins within hours of overdose, and consists of nausea, vomiting, a pale appearance, and sweating.[10] However, patients often have no specific symptoms or only mild symptoms in the first 24 hours of poisoning. Rarely, after massive overdoses, patients may develop symptoms of metabolic acidosis and coma early in the course of poisoning.[11][12]
The second phase occurs between 24 hours and 72 hours following overdose and consists of signs of increasing liver damage. In general, damage occurs in liver cells as they metabolize the paracetamol. Hallmark pathology on liver biopsy includes regions of coagulative necrosis in zone 3 of the liver acinus, around the central venules, as these hepatocytes have higher concentrations of cytochrome P450 enzymes compared to zone 1 hepatocytes surrounding the portal venule of the acinus. Remaining viable hepatocytes frequently show ballooning injury and steatosis.[13] The individual may experience right upper quadrant abdominal pain. The increasing liver damage also changes biochemical markers of liver function; International normalized ratio (INR) and the liver transaminases ALT and AST rise to abnormal levels.[14] Acute kidney failure may also occur during this phase, typically caused by either hepatorenal syndrome or multiple organ dysfunction syndrome. In some cases, acute kidney failure may be the primary clinical manifestation of toxicity. In these cases, it has been suggested that the toxic metabolite is produced more in the kidneys than in the liver.[15]
The third phase follows at 3 to 5 days, and is marked by complications of massive liver necrosis leading to fulminant liver failure with complications of coagulation defects, low blood sugar, kidney failure, hepatic encephalopathy, brain swelling, sepsis, multiple organ failure, and death.[10] If the third phase is survived, the liver necrosis runs its course, and liver and kidney function typically return to normal in a few weeks.[16] The severity of paracetamol toxicity varies depending on the dose and whether appropriate treatment is received.
Cause
[edit]The toxic dose of paracetamol is highly variable. In general the recommended maximum daily dose for healthy adults is 4 grams.[17][18] Higher doses lead to increasing risk of toxicity. In adults, single doses above 10 grams or 200 mg/kg of bodyweight, whichever is lower, have a reasonable likelihood of causing toxicity.[19][20] Toxicity can also occur when multiple smaller doses within 24 hours exceed these levels.[20] Following a dose of 1 gram of paracetamol four times a day for two weeks, patients can expect an increase in alanine transaminase in their liver to typically about three times the normal value.[21] It is unlikely that this dose would lead to liver failure.[22] Studies have shown significant hepatotoxicity is uncommon in patients who have taken greater than normal doses over 3 to 4 days.[23] In adults, a dose of 6 grams a day over the preceding 48 hours could potentially lead to toxicity,[20] while in children acute doses above 200 mg/kg could potentially cause toxicity.[24] Acute paracetamol overdose in children rarely causes illness or death, and it is very uncommon for children to have levels that require treatment, with chronic larger-than-normal doses being the major cause of toxicity in children.[20]
Intentional overdosing (self-poisoning, with suicidal intent) is frequently implicated in paracetamol toxicity.[25] In a 2006 review, paracetamol was the most frequently ingested compound in intentional overdosing.[26]
In rare individuals, paracetamol toxicity can result from normal use.[27] This may be due to individual ("idiosyncratic") differences in the expression and activity of certain enzymes in one of the metabolic pathways that handle paracetamol (see paracetamol's metabolism).
Risk factors
[edit]A number of factors can potentially increase the risk of developing paracetamol toxicity. Chronic excessive alcohol consumption can induce CYP2E1, thus increasing the potential toxicity of paracetamol. In one study of patients with liver injury, 64% reported alcohol intakes of greater than 80 grams a day, while 35% took 60 grams a day or less.[28] Whether chronic alcoholism should be considered a risk factor has been debated by some clinical toxicologists.[29][30] For chronic alcohol users, acute alcohol ingestion at the time of a paracetamol overdose may have a protective effect.[29][31] For non-chronic alcohol users, acute alcohol consumption had no protective effect.
Fasting is a risk factor, possibly because of depletion of liver glutathione reserves.[20] The concomitant use of the CYP2E1 inducer isoniazid increases the risk of hepatotoxicity, though whether 2E1 induction is related to the hepatotoxicity in this case is unclear.[32][33] Concomitant use of other drugs that induce CYP enzymes, such as antiepileptics including carbamazepine, phenytoin, and barbiturates, have also been reported as risk factors.[34]
Pathophysiology
[edit]
When taken in normal therapeutic doses, paracetamol has been shown to be safe.[14] Following a therapeutic dose, it is mostly converted to nontoxic metabolites via Phase II metabolism by conjugation with sulfate and glucuronide, with a small portion being oxidized via the cytochrome P450 enzyme system.[35] Cytochromes P450 2E1 and 3A4 convert approximately 5% of paracetamol to a highly reactive intermediary metabolite, N-acetyl-p-benzoquinone imine (NAPQI).[35][14][36][37][38] Under normal conditions, NAPQI is detoxified by conjugation with glutathione to form cysteine and mercapturic acid conjugates.[35][39]
In cases of paracetamol overdose, the sulfate and glucuronide pathways become saturated, and more paracetamol is shunted to the cytochrome P450 system to produce NAPQI. As a result, hepatocellular supplies of glutathione become depleted, as the demand for glutathione is higher than its regeneration.[39] NAPQI therefore remains in its toxic form in the liver and reacts with cellular membrane molecules, resulting in widespread hepatocyte damage and death, leading to acute liver necrosis.[35][40] In animal studies, the liver's stores of glutathione must be depleted to less than 70% of normal levels before liver toxicity occurs.[36]
Diagnosis
[edit]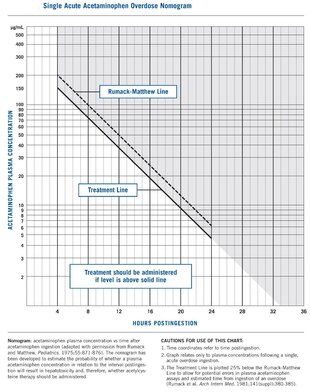
A person's history of taking paracetamol is somewhat accurate for the diagnosis.[41] The most effective way to diagnose poisoning is by obtaining a blood paracetamol level. A drug nomogram developed in 1975, called the Rumack–Matthew nomogram, estimates the risk of toxicity based on the serum concentration of paracetamol at a given number of hours after ingestion.[10] To determine the risk of potential hepatotoxicity, the paracetamol level is traced along the nomogram. Use of a timed serum paracetamol level plotted on the nomogram appears to be the best marker indicating the potential for liver injury.[20] A paracetamol level drawn in the first four hours after ingestion may underestimate the amount in the system because paracetamol may still be in the process of being absorbed from the gastrointestinal tract. Therefore, a serum level taken before 4 hours is not recommended.[19]
Clinical or biochemical evidence of liver toxicity may develop in one to four days, although, in severe cases, it may be evident in 12 hours.[42] Right-upper-quadrant tenderness may be present and can aid in diagnosis. Laboratory studies may show evidence of liver necrosis with elevated AST, ALT, bilirubin, and prolonged coagulation times, particularly an elevated prothrombin time.[43] After paracetamol overdose, when AST and ALT exceed 1000 IU/L, paracetamol-induced hepatotoxicity can be diagnosed.[42] In some cases, the AST and ALT levels can exceed 10,000 IU/L.[44]
Detection in body fluids
[edit]Paracetamol may be quantified in blood, plasma, or urine as a diagnostic tool in clinical poisoning situations or to aid in the medicolegal investigation of suspicious deaths. The concentration in serum after a typical dose of paracetamol usually peaks below 30 mg/L, which equals 200 μmol/L.[45] Levels of 30–300 mg/L (200–2000 μmol/L) are often observed in overdose patients. Postmortem blood levels have ranged from 50 to 400 mg/L in persons dying due to acute overdosage. Automated colorimetric techniques, gas chromatography and liquid chromatography are currently in use for the laboratory analysis of the drug in physiological specimens.[46][47]
Prevention
[edit]
Limitation of availability
[edit]Limiting the availability of paracetamol tablets has been attempted in some countries. In the UK, sales of over-the-counter paracetamol are restricted to packs of 32 × 500 mg tablets in pharmacies, and 16 × 500 mg tablets in non-pharmacy outlets. Pharmacists may provide up to 100 tablets for those with chronic conditions at the pharmacist's discretion.[48][49] In Ireland, the limits are 24 and 12 tablets, respectively.[50] Subsequent study suggests that the reduced availability in large numbers had a significant effect in reducing poisoning deaths from paracetamol overdose.[51]
One suggested method of prevention is to make paracetamol a prescription-only medicine, or to remove it entirely from the market. However, overdose is a relatively minor problem; for example, 0.08% of the UK population (over 50 thousand people) present with paracetamol overdose each year. In contrast, paracetamol is a safe and effective medication that is taken without complications by millions of people.[52] In addition, alternative pain relief medications such as aspirin are more toxic in overdose, whereas non-steroidal anti-inflammatory drugs are associated with more adverse effects following normal use.[53]
Combination with other agents
[edit]One strategy for reducing harm done by acetaminophen overdoses is selling paracetamol pre-combined in tablets either with an emetic[52] or an antidote. Paradote was a tablet sold in the UK which combined 500 mg paracetamol with 100 mg methionine,[54] an amino acid formerly[20] used in the treatment of paracetamol overdose.
There have been no studies so far on the effectiveness of paracetamol when given in combination with its most commonly used antidote, acetylcysteine.[55]
Calcitriol, the active metabolite of vitamin D3, appears to be a catalyst for glutathione production.[56] Calcitriol was found to increase glutathione levels in rat astrocyte primary cultures on average by 42%, increasing glutathione protein concentrations from 29 nmol/mg to 41 nmol/mg, 24 and 48 hours after administration; it continued to have an influence on glutathione levels 96 hours after administration.[57] It has been proposed that co-administration of calcitriol, via injection, may improve treatment outcomes.
Paracetamol replacements
[edit]Paracetamol ester prodrug containing L-pyroglutamic acid (PGA), a biosynthetic precursor of glutathione, has been synthesized to reduce paracetamol hepatotoxicity and improve bioavailability. The toxicological studies of different paracetamol esters show that L-5-oxo-pyrrolidine-2-paracetamol carboxylate reduces toxicity after administration of an overdose of paracetamol to mice. The liver glutathione values in mice induced by intraperitoneal injection of the ester are superimposable with the GSH levels recorded in untreated mice control group. The mice group treated with an equivalent dose of paracetamol showed a significant decrease of glutathione of 35% (p<0.01 vs untreated control group). The oral LD50 was found to be greater than 2000 mg kg-1, whereas the intraperitoneal LD50 was 1900 mg kg-1. These results taken together with the good hydrolysis and bioavailability data show that this ester is a potential candidate as a prodrug of paracetamol.[58]
Treatment
[edit]Gastrointestinal decontamination
[edit]In adults, the initial treatment for paracetamol overdose is gastrointestinal decontamination. Paracetamol absorption from the gastrointestinal tract is complete within two hours under normal circumstances, so decontamination is most helpful if performed within this timeframe. Gastric lavage, better known as stomach pumping, may be considered if the amount ingested is potentially life-threatening and the procedure can be performed within 60 minutes of ingestion.[59] Administration of activated charcoal is the most common gastrointestinal decontamination procedure as it efficiently adsorbs paracetamol, thereby reducing its gastrointestinal absorption.[60][61] Administering activated charcoal also poses less risk of aspiration than gastric lavage.[62]
It appears that the most benefit from activated charcoal is gained if it is given within 30 minutes to two hours of ingestion.[63][62] Administering activated charcoal later than 2 hours can be considered in patients that may have delayed gastric emptying due to co-ingested drugs or following ingestion of sustained- or delayed-release paracetamol preparations. Activated charcoal should also be administered if co-ingested drugs warrant decontamination.[42] There was reluctance to give activated charcoal in paracetamol overdose, because of the concern that it may also absorb the oral antidote acetylcysteine.[64] Studies have shown that 39% less acetylcysteine is absorbed into the body when they are administered together.[65] There are conflicting recommendations regarding whether to change the dosing of oral acetylcysteine after the administration of activated charcoal, and even whether the dosing of acetylcysteine needs to be altered at all.[65][66] Intravenous acetylcysteine has no interaction with activated charcoal.
Inducing vomiting with syrup of ipecac has no role in paracetamol overdose because the vomiting it induces delays the effective administration of activated charcoal and oral acetylcysteine.[19] Liver injury is extremely rare after acute accidental ingestion in children under 6 years of age. Children with accidental exposures do not require gastrointestinal decontamination with either gastric lavage, activated charcoal, or syrup of ipecac.[20]
Acetylcysteine
[edit]
Acetylcysteine, also called N-acetylcysteine or NAC, works to reduce paracetamol toxicity by replenishing body stores of the antioxidant glutathione. Glutathione reacts with the toxic NAPQI metabolite so that it does not damage cells and can be safely excreted.[67] NAC was usually given following a treatment nomogram (one for patients with risk factors, and one for those without) but the use of the nomogram is no longer recommended as the evidence base to support the use of risk factors was poor and inconsistent and many of the risk factors are imprecise and difficult to determine with sufficient certainty in clinical practice.[68] Cysteamine and methionine have also been used to prevent hepatotoxicity,[69] although studies show that both are associated with more adverse effects than acetylcysteine.[20] Additionally, acetylcysteine has been shown to be a more effective antidote, particularly in patients presenting greater than 8 hours post-ingestion[70] and for those who present with liver failure symptoms.[61]
If the person presents less than eight hours after paracetamol overdose, then acetylcysteine significantly reduces the risk of serious hepatotoxicity and guarantees survival.[20] If acetylcysteine is started more than 8 hours after ingestion, there is a sharp decline in its effectiveness because the cascade of toxic events in the liver has already begun, and the risk of acute liver necrosis and death increases dramatically. Although acetylcysteine is most effective if given early, it still has beneficial effects if given as late as 48 hours after ingestion.[71] If the person presents more than eight hours after the paracetamol overdose, then activated charcoal is not useful, and acetylcysteine is started immediately. In earlier presentations, charcoal can be given when the patient arrives and acetylcysteine is initiated while waiting for the paracetamol level results to return from the laboratory.[20]
In United States practice, intravenous (IV) and oral administration are considered to be equally effective and safe if given within 8 hours of ingestion.[72][73] However, IV is the only recommended route in Australasian and British practice.[20][74] Oral acetylcysteine is given as a 140 mg/kg loading dose followed by 70 mg/kg every four hours for 17 more doses, and if the patient vomits within 1 hour of dose, the dose must be repeated.[75][76] Oral acetylcysteine may be poorly tolerated due to its unpleasant taste, odor, and its tendency to cause nausea and vomiting.[72] If repeated doses of charcoal are indicated because of another ingested drug, then subsequent doses of charcoal and acetylcysteine should be staggered.[42]
Intravenous acetylcysteine is given as a continuous infusion over 20 hours for a total dose 300 mg/kg. Recommended administration involves infusion of a 150 mg/kg loading dose over 15 to 60 minutes, followed by a 50 mg/kg infusion over four hours; the last 100 mg/kg are infused over the remaining 16 hours of the protocol.[20] Intravenous acetylcysteine has the advantage of shortening hospital stay, increasing both doctor and patient convenience, and allowing administration of activated charcoal to reduce absorption of both the paracetamol and any co-ingested drugs without concerns about interference with oral acetylcysteine.[77][needs update] Intravenous dosing varies with weight, specifically in children. For patients less than 20 kg, the loading dose is 150 mg/kg in 3 mL/kg diluent, administered over 60 minutes; the second dose is 50 mg/kg in 7 mL/kg diluent over 4 hours; and the third and final dose is 100 mg/kg in 14 mL/kg diluent over 16 hours.[76] Because of the risk of adverse events, electrolyte derangements and fluid shifts associated with larger doses of acetylcysteine dose capping regimens have been suggested. To date no increased risk of hepatic injury or failure has been noted with this dose capping strategy.[78]
The most common adverse effect to acetylcysteine treatment is an anaphylactoid reaction, usually manifested by rash, wheeze, or mild hypotension. Adverse reactions are more common in people treated with IV acetylcysteine, occurring in up to 20% of patients.[79][80] Anaphylactoid reactions are more likely to occur with the first infusion (the loading dose).[79] Rarely, severe life-threatening reactions may occur in predisposed individuals, such as patients with asthma or atopic dermatitis, and may be characterized by respiratory distress, facial swelling, and even death.[79][81][82]
If an anaphylactoid reaction occurs the acetylcysteine is temporarily halted or slowed and antihistamines and other supportive care is administered.[79][83][84] For example, a nebulised beta-agonist like salbutamol may be indicated in the event of significant bronchospasm (or prophylactically in patients with a history of bronchospasm secondary to acetylcysteine). It is also important to closely monitor fluids and electrolytes.[79]
Liver transplant
[edit]In people who develop acute liver failure or who are otherwise expected to die from liver failure, the mainstay of management is liver transplantation.[52] Liver transplants are performed in specialist centers. The most commonly used criteria for liver transplant were developed by physicians at King's College Hospital in London. Patients are recommended for transplant if they have an arterial blood pH less than 7.3 after fluid resuscitation or if a patient has Grade III or IV encephalopathy, a prothrombin time greater than 100 seconds, and a serum creatinine greater than 300 mmol/L In a 24-hour period.[85] Other forms of liver support have been used including partial liver transplants. These techniques have the advantage of supporting the patient while their own liver regenerates. Once liver function returns immunosuppressive drugs are commenced and they have to take immunosuppressive medication for the rest of their lives.[86][87]
Prognosis
[edit]The mortality rate from paracetamol overdose increases two days after the ingestion, reaches a maximum on day four, and then gradually decreases. Acidosis is the most important single indicator of probable mortality and the need for transplantation. A mortality rate of 95% without transplant was reported in patients who had a documented pH less than 7.30. Other indicators of poor prognosis include chronic kidney disease (stage 3 or worse), hepatic encephalopathy, a markedly elevated prothrombin time, or an elevated blood lactic acid level (lactic acidosis).[85][88] One study has shown that a factor V level less than 10% of normal indicated a poor prognosis (91% mortality), whereas a ratio of factor VIII to factor V of less than 30 indicated a good prognosis (100% survival).[89] Patients with a poor prognosis are usually identified for likely liver transplantation.[85] Patients that do not die are expected to fully recover and have a normal life expectancy and quality of life.[90] Fertility may deteriorate. There is evidence of acetaminophen gonadotoxicity and a persistent negative effect of poisoning on the ability to conceive due to deterioration in sperm quality (impaired sperm morphology) in a previously healthy person. [citation needed]
Epidemiology
[edit]Many over-the-counter and prescription-only medications contain paracetamol. Because of its wide availability paired with comparably high toxicity, (compared to ibuprofen and aspirin) there is a much higher potential for overdose.[91] Paracetamol toxicity is one of the most common causes of poisoning worldwide.[25] In the United States, the United Kingdom, Australia, and New Zealand, paracetamol is the most common cause of drug overdoses.[20][92][93] Additionally, in both the United States and the United Kingdom it is the most common cause of acute liver failure.[94][9]
In England and Wales an estimated 41,200 cases of paracetamol poisoning occurred in 1989 to 1990, with a mortality of 0.40%. It is estimated that 150 to 200 deaths and 15 to 20 liver transplants occur as a result of poisoning each year in England and Wales.[80] Paracetamol overdose results in more calls to poison control centers in the US than overdose of any other pharmacological substance, accounting for more than 100,000 calls, as well as 56,000 emergency room visits, 2,600 hospitalizations, and 458 deaths due to acute liver failure per year.[95] A study of cases of acute liver failure between November 2000 and October 2004 by the Centers for Disease Control and Prevention in the US found that paracetamol was the cause of 41% of all cases in adults, and 25% of cases in children.[96]
References
[edit]- ^ a b c d e f g h i j k l m n o p q r Ferri FF (2016). Ferri's Clinical Advisor 2017 E-Book: 5 Books in 1. Elsevier Health Sciences. p. 11. ISBN 978-0-323-44838-3. Archived from the original on September 10, 2017. Retrieved July 6, 2017.
- ^ a b Woolley D, Woolley A (2017). Practical Toxicology: Evaluation, Prediction, and Risk, Third Edition. CRC Press. p. 330. ISBN 978-1-4987-0930-9. Archived from the original on September 10, 2017. Retrieved July 5, 2017.
- ^ Proudfoot AT, Wright N (September 5, 1970). "Acute Paracetamol Poisoning". BMJ. 3 (5722): 557–558. doi:10.1136/bmj.3.5722.557. PMC 1701561. PMID 5311516.
- ^ Ferner RE, Dear JW, Bateman DN (April 19, 2011). "Management of paracetamol poisoning". BMJ. 342 (apr19 2): d2218. doi:10.1136/bmj.d2218. PMID 21508044. S2CID 5339635.
- ^ Chun L.J., Tong M.J., Busuttil R.W., Hiatt J.R. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol. 2009;43:342–349.
- ^ a b c d e Webb A, Gattinoni L (2016). Oxford Textbook of Critical Care. Oxford University Press. p. 1518. ISBN 978-0-19-960083-0. Archived from the original on September 10, 2017. Retrieved July 6, 2017.
- ^ a b Prout J, Jones T, Martin D (2014). Advanced Training in Anaesthesia. OUP Oxford. p. 166. ISBN 978-0-19-151177-6. Archived from the original on September 10, 2017.
- ^ Yamada T (2011). Textbook of Gastroenterology. John Wiley & Sons. p. PT4008. ISBN 978-1-4443-5941-1. Archived from the original on September 10, 2017.
- ^ a b Ryder SD, Beckingham IJ (February 2001). "Other causes of parenchymal liver disease". BMJ (Clinical Research Ed.). 322 (7281): 290–2. doi:10.1136/bmj.322.7281.290. PMC 1119531. PMID 11157536.
- ^ a b c Rumack B, Matthew H (1975). "Acetaminophen poisoning and toxicity". Pediatrics. 55 (6): 871–76. doi:10.1542/peds.55.6.871. PMID 1134886. S2CID 45739342.
- ^ Zezulka A, Wright N (September 1982). "Severe metabolic acidosis early in paracetamol poisoning". British Medical Journal (Clinical Research Ed.). 285 (6345): 851–2. doi:10.1136/bmj.285.6345.851. PMC 1499688. PMID 6811039.
- ^ Roth B, Woo O, Blanc P (April 1999). "Early metabolic acidosis and coma after acetaminophen ingestion". Annals of Emergency Medicine. 33 (4): 452–6. doi:10.1016/S0196-0644(99)70312-4. PMID 10092726.
- ^ "Acute Hepatic Necrosis". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury (Internet). National Institute of Diabetes and Digestive and Kidney Diseases. May 4, 2019. PMID 31689035. Retrieved November 19, 2023.
- ^ a b c Heard KJ (July 2008). "Acetylcysteine for Acetaminophen Poisoning". The New England Journal of Medicine. 359 (3): 285–92. doi:10.1056/NEJMct0708278. PMC 2637612. PMID 18635433.
- ^ Boutis K, Shannon M (2001). "Nephrotoxicity after acute severe acetaminophen poisoning in adolescents". Journal of Toxicology: Clinical Toxicology. 39 (5): 441–5. doi:10.1081/CLT-100105413. PMID 11545233. S2CID 35456821.
- ^ Linden CH, Rumack BH (February 1984). "Acetaminophen overdose". Emergency Medicine Clinics of North America. 2 (1): 103–19. doi:10.1016/S0733-8627(20)30837-3. PMID 6394298.
- ^ Research Cf. "Drug Safety and Availability - Notice to Industry: Final Guidance for Over-the-Counter Products that Contain Acetaminophen". www.fda.gov. Archived from the original on July 22, 2017. Retrieved August 22, 2017.
- ^ "Paracetamol for adults: painkiller to treat aches, pains and fever - NHS.UK". NHS.UK. Archived from the original on August 22, 2017. Retrieved August 22, 2017.
- ^ a b c Dart RC, Erdman AR, Olson KR, Christianson G, Manoguerra AS, Chyka PA, Caravati EM, Wax PM, Keyes DC, Woolf AD, Scharman EJ, Booze LL, Troutman WG; American Association of Poison Control Centers (2006). "Acetaminophen poisoning: an evidence-based consensus guideline for out-of-hospital management". Clinical Toxicology. 44 (1): 1–18. doi:10.1080/15563650500394571. PMID 16496488.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k l m n Daly FF, Fountain JS, Murray L, Graudins A, Buckley NA (March 2008). "Guidelines for the management of paracetamol poisoning in Australia and New Zealand—explanation and elaboration. A consensus statement from clinical toxicologists consulting to the Australasian poisons information centres". The Medical Journal of Australia. 188 (5): 296–301. doi:10.5694/j.1326-5377.2008.tb01625.x. PMID 18312195. S2CID 9505802. Archived from the original on July 23, 2008.
- ^ Watkins PB, Kaplowitz N, Slattery JT, et al. (July 2006). "Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial". JAMA: The Journal of the American Medical Association. 296 (1): 87–93. doi:10.1001/jama.296.1.87. PMID 16820551.
- ^ Dart RC, Bailey E (2007). "Does therapeutic use of acetaminophen cause acute liver failure?". Pharmacotherapy. 27 (9): 1219–30. doi:10.1592/phco.27.9.1219. PMID 17723075. S2CID 10493231.
- ^ Daly FF, O'Malley GF, Heard K, Bogdan GM, Dart RC (October 2004). "Prospective evaluation of repeated supratherapeutic acetaminophen (paracetamol) ingestion". Annals of Emergency Medicine. 44 (4): 393–8. doi:10.1016/j.annemergmed.2004.05.005. PMID 15459622.
- ^ Tenenbein M (2004). "Acetaminophen: the 150 mg/kg myth". Journal of Toxicology: Clinical Toxicology. 42 (2): 145–8. doi:10.1081/CLT-120030939. PMID 15214618. S2CID 29253361.
- ^ a b Gunnell D, Murray V, Hawton K (2000). "Use of paracetamol (acetaminophen) for suicide and nonfatal poisoning: worldwide patterns of use and misuse". Suicide and Life-Threatening Behavior. 30 (4): 313–26. doi:10.1111/j.1943-278X.2000.tb01098.x. PMID 11210057.
- ^ Kapur N, Turnbull P, Hawton K, Simkin S, Mackway-Jones K, Gunnel D (June 2006). "The Hospital Management of Fatal Self-Poisoning in Industrialized Countries: An Opportunity for Suicide Prevention?". Suicide and Life-Threatening Behavior. 36 (3): 302–12. doi:10.1521/suli.2006.36.3.302. PMID 16805658.
- ^ Vuppalanchi R, Liangpunsakul S, Chalasani N (March 2007). "Etiology of new-onset jaundice: how often is it caused by idiosyncratic drug-induced liver injury in the United States?". Am. J. Gastroenterol. 102 (3): 558–62, quiz 693. doi:10.1111/j.1572-0241.2006.01019.x. PMID 17156142. S2CID 23813443.
- ^ Zimmerman HJ, Maddrey WC (1995). "Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure". Hepatology. 22 (3): 767–73. doi:10.1002/hep.1840220312. PMID 7657281. S2CID 24215641.
- ^ a b Dargan PI, Jones AL (2002). "Should a lower treatment line be used when treating paracetamol poisoning in patients with chronic alcoholism?: a case against". Drug Safety. 25 (9): 625–32. doi:10.2165/00002018-200225090-00002. PMID 12137557. S2CID 36470507.
- ^ Buckley NA, Srinivasan J (2002). "Should a lower treatment line be used when treating paracetamol poisoning in patients with chronic alcoholism?: a case for". Drug Safety. 25 (9): 619–24. doi:10.2165/00002018-200225090-00001. PMID 12137556. S2CID 10343543.
- ^ Schmidt LE, Dalhoff K, Poulsen HE (April 2002). "Acute versus chronic alcohol consumption in acetaminophen-induced hepatotoxicity". Hepatology. 35 (4): 876–82. doi:10.1053/jhep.2002.32148. PMID 11915034. S2CID 38354674.
- ^ Crippin JS (April 1993). "Acetaminophen hepatotoxicity: potentiation by isoniazid". The American Journal of Gastroenterology. 88 (4): 590–2. PMID 8470644.
- ^ Nolan CM, Sandblom RE, Thummel KE, Slattery JT, Nelson SD (1994). "Hepatotoxicity associated with acetaminophen usage in patients receiving multiple drug therapy for tuberculosis". Chest. 105 (2): 408–11. doi:10.1378/chest.105.2.408. PMID 7508362.
- ^ Bray GP, Harrison PM, O'Grady JG, Tredger JM, Williams R (July 1992). "Long-term anticonvulsant therapy worsens outcome in paracetamol-induced fulminant hepatic failure". Human & Experimental Toxicology. 11 (4): 265–70. Bibcode:1992HETox..11..265B. doi:10.1177/096032719201100405. PMID 1354974. S2CID 34093648.
- ^ a b c d Metabolism of acetaminophen (paracetamol), acetanilide and phenacetin Archived August 30, 2012, at the Wayback Machine
- ^ a b Richardson, JA (July–September 2000). "Management of acetaminophen and ibuprofen toxicoses in dogs and cats" (PDF). Journal of Veterinary Emergency and Critical Care. 10 (4): 285–291. doi:10.1111/j.1476-4431.2000.tb00013.x. Archived from the original (PDF) on November 22, 2008.
- ^ Rumbeiha WK, Lin YS, Oehme FW (November 1995). "Comparison of N-acetylcysteine and methylene blue, alone or in combination, for treatment of acetaminophen toxicosis in cats". American Journal of Veterinary Research. 56 (11): 1529–33. doi:10.2460/ajvr.1995.56.11.1529. PMID 8585668.
- ^ Corcoran GB, Mitchell JR, Vaishnav YN, Horning EC (November 1980). "Evidence that acetaminophen and N-hydroxyacetaminophen form a common arylating intermediate, N-acetyl-p-benzoquinoneimine". Molecular Pharmacology. 18 (3): 536–42. PMID 7464816.
- ^ a b Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB (October 1973). "Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione". The Journal of Pharmacology and Experimental Therapeutics. 187 (1): 211–7. PMID 4746329.
- ^ Dai Y, Cederbaum AI (June 1995). "Cytotoxicity of acetaminophen in human cytochrome P4502E1-transfected HepG2 cells". The Journal of Pharmacology and Experimental Therapeutics. 273 (3): 1497–505. PMID 7791125.
- ^ Camilleri R (June 2015). "A meta-analysis of the reliability of the history in suspected poisoning". The Journal of Emergency Medicine. 48 (6): 679–84. doi:10.1016/j.jemermed.2014.12.067. PMID 25827782.
- ^ a b c d Farrell SE (October 3, 2007). "Toxicity, Acetaminophen". emedicine. Archived from the original on October 29, 2008. Retrieved November 9, 2008.
- ^ Bartlett D (June 2004). "Acetaminophen toxicity". Journal of Emergency Nursing. 30 (3): 281–3. doi:10.1016/j.jen.2004.01.023. PMID 15192687.
- ^ Jones AL (March 2000). "Recent advances in the management of late paracetamol poisoning". Emergency Medicine Australasia. 12 (1): 14–21. doi:10.1046/j.1442-2026.2000.00088.x.
- ^ Marx J, Walls R, Hockberger R (2013). Rosen's Emergency Medicine - Concepts and Clinical Practice. Elsevier Health Sciences. ISBN 978-1-4557-4987-4.
- ^ Shihana F, Dissanayake D, Dargan P, Dawson A (2010). "A modified low-cost colorimetric method for paracetamol (acetaminophen) measurement in plasma". Clin Toxicol. 48 (1): 42–46. doi:10.3109/15563650903443137. PMC 3145116. PMID 20095813.
- ^ Baselt R (2011). Disposition of Toxic Drugs and Chemicals in Man (9th ed.). Seal Beach, California: Biomedical Publications. pp. 9–12.
- ^ Hughes B, Durran A, Langford NJ, Mutimer D (August 2003). "Paracetamol poisoning—impact of pack size restrictions". Journal of Clinical Pharmacy and Therapeutics. 28 (4): 307–10. doi:10.1046/j.1365-2710.2003.00497.x. PMID 12911683. S2CID 29408778.
- ^ Sheen CL, Dillon JF, Bateman DN, Simpson KJ, Macdonald TM (September 2002). "Paracetamol toxicity: epidemiology, prevention and costs to the health-care system". QJM: Monthly Journal of the Association of Physicians. 95 (9): 609–19. doi:10.1093/qjmed/95.9.609. PMID 12205339.
- ^ Laffoy M, Scallan E, Byrne G (2001). "Paracetamol availability and overdose in Ireland". Irish Medical Journal. 94 (7): 212–4. PMID 11693213.
- ^ Gunnell D, Hawton K, Bennewith O, Cooper J, Simkin S, Donovan J, Evans J, Longson D, O'Connor S, Kapur N (October 2013). "3. Studies to evaluate the impact of the 1998 UK legislation restricting pack sizes of paracetamol". A multicentre programme of clinical and public health research in support of the National Suicide Prevention Strategy for England. NIHR Journals Library.
- ^ a b c Dargan PI, Jones AL (April 2003). "Management of paracetamol poisoning". Trends in Pharmacological Sciences. 24 (4): 154–7. doi:10.1016/S0165-6147(03)00053-1. PMID 12706999.
- ^ Jones A (2002). "Over-the-counter analgesics: a toxicology perspective". Am J Ther. 9 (3): 245–57. doi:10.1097/00045391-200205000-00010. PMID 11941384. S2CID 25957761.
- ^ Heptonstall JP (April 2006). "Time to make paracetamol with methionine available". BMJ (Clinical Research Ed.). 332 (7544): 795. doi:10.1136/bmj.332.7544.795-b. PMC 1420701. PMID 16575097.
- ^ Chang M. "Acetaminophen in Combination With N-Acetylcysteine (NAC) Versus Placebo in Treating Fever". Archived from the original on October 19, 2012. Retrieved November 17, 2012.
- ^ Garcion E, Wion-Barbot N, Montero-Menei C, Berger F, Wion D (2002). "New clues about vitamin D functions in the nervous system". Trends in Endocrinology and Metabolism. 13 (3): 100–5. doi:10.1016/S1043-2760(01)00547-1. PMID 11893522. S2CID 19010892.
- ^ Garcion E, Sindji L, Leblondel G, Brachet P, Darcy F (2002). "1,25-Dihydroxyvitamin D3 Regulates the Synthesis of γ-Glutamyl Transpeptidase and Glutathione Levels in Rat Primary Astrocytes". Journal of Neurochemistry. 73 (2): 859–866. doi:10.1046/j.1471-4159.1999.0730859.x. PMID 10428085. S2CID 29314065.
- ^ Bousquet E, Marrazzo A, Puglisi G, Spadaro A (1996). "Synthesis, physical properties, toxicological studies and bioavailability of L-pyroglutamic and L-glutamic acid esters of paracetamol as potentially useful prodrugs". J Pharm Pharmacol. 48 (5): 479–85. doi:10.1111/j.2042-7158.1996.tb05958.x. PMID 8799871. S2CID 38408242.
- ^ Vale JA, Kulig K; American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists (2004). "Position paper: gastric lavage". Journal of Toxicology: Clinical Toxicology. 42 (7): 933–43. doi:10.1081/CLT-200045006. PMID 15641639. S2CID 29957973.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Spiller HA, Sawyer TS (August 2007). "Impact of activated charcoal after acute acetaminophen overdoses treated with N-acetylcysteine". The Journal of Emergency Medicine. 33 (2): 141–4. doi:10.1016/j.jemermed.2007.02.016. PMID 17692765.
- ^ a b Chiew AL, Gluud C, Brok J, Buckley NA (February 23, 2018). "Interventions for paracetamol (acetaminophen) overdose". The Cochrane Database of Systematic Reviews. 2018 (2): CD003328. doi:10.1002/14651858.CD003328.pub3. PMC 6491303. PMID 29473717.
- ^ a b Buckley NA, Whyte IM, O'Connell DL, Dawson AH (1999). "Activated charcoal reduces the need for N-acetylcysteine treatment after acetaminophen (paracetamol) overdose". Journal of Toxicology: Clinical Toxicology. 37 (6): 753–7. doi:10.1081/CLT-100102452. PMID 10584587.
- ^ Isbister G, Whyte I, Dawson A (2001). "Pediatric acetaminophen overdose". Journal of Toxicology: Clinical Toxicology. 39 (2): 169–72. doi:10.1081/CLT-100103834. PMID 11407504. S2CID 40144977.
- ^ Renzi FP, Donovan JW, Martin TG, Morgan L, Harrison EF (June 1985). "Concomitant use of activated charcoal and N-acetylcysteine". Annals of Emergency Medicine. 14 (6): 568–72. doi:10.1016/S0196-0644(85)80781-2. PMID 3994080.
- ^ a b Ekins BR, Ford DC, Thompson MI, Bridges RR, Rollins DE, Jenkins RD (November 1987). "The effect of activated charcoal on N-acetylcysteine absorption in normal subjects". The American Journal of Emergency Medicine. 5 (6): 483–7. doi:10.1016/0735-6757(87)90166-5. PMID 3663288.
- ^ Spiller HA, Krenzelok EP, Grande GA, Safir EF, Diamond JJ (March 1994). "A prospective evaluation of the effect of activated charcoal before oral N-acetylcysteine in acetaminophen overdose". Annals of Emergency Medicine. 23 (3): 519–23. doi:10.1016/S0196-0644(94)70071-0. PMID 8135427.
- ^ Piperno E, Berssenbruegge DA (October 1976). "Reversal of experimental paracetamol toxicosis with N-acetylcysteine". Lancet. 2 (7988): 738–9. doi:10.1016/S0140-6736(76)90030-1. PMID 61415. S2CID 34716320.
- ^ "Paracetamol overdose: new guidance on treatment with intravenous acetylcysteine". Drug Safety Update. 6 (2): A1. September 2012. Archived from the original on October 27, 2012.
- ^ Mant TG, Tempowski JH, Volans GN, Talbot JC (July 1984). "Adverse reactions to acetylcysteine and effects of overdose". British Medical Journal (Clinical Research Ed.). 289 (6439): 217–9. doi:10.1136/bmj.289.6439.217. PMC 1442311. PMID 6234965.
- ^ Alsalim W, Fadel M (July 2003). "Oral methionine compared with intravenous n-acetyl cysteine for paracetamol overdose". Emergency Medicine Journal. 20 (4): 366–7. doi:10.1136/emj.20.4.366. PMC 1726135. PMID 12835357.
- ^ Keays R, Harrison P, Wendon J, Forbes A, Gove C, Alexander G, Williams R (1991). "Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial". BMJ. 303 (6809): 1026–9. doi:10.1136/bmj.303.6809.1026. PMC 1671790. PMID 1954453.
- ^ a b Kanter MZ (October 2006). "Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning". American Journal of Health-System Pharmacy. 63 (19): 1821–7. doi:10.2146/ajhp060050. PMID 16990628.
- ^ Schwarz E, Cohn B (2014). "Is Intravenous Acetylcysteine More Effective Than Oral Administration for the Prevention of Hepatotoxicity in Acetaminophen Overdose?". Annals of Emergency Medicine. 63 (1): 79–80. doi:10.1016/j.annemergmed.2013.07.002. PMID 23927960.
- ^ Selvan VA, Calvert SH, Cavell G, Glucksman E, Kerins M, Gonzalez J (July 2007). "Weight-based N-acetylcysteine dosing chart to minimise the risk of calculation errors in prescribing and preparing N-acetylcysteine infusions for adults presenting with paracetamol overdose in the emergency department". Emergency Medicine Journal. 24 (7): 482–4. doi:10.1136/emj.2006.043141. PMC 2796160. PMID 17582039.
- ^ Woo OF, Mueller PD, Olson KR, Anderson IB, Kim SY (April 2000). "Shorter duration of oral N-acetylcysteine therapy for acute acetaminophen overdose". Annals of Emergency Medicine. 35 (4): 363–8. doi:10.1016/S0196-0644(00)70055-2. PMID 10736123.
- ^ a b "Acetaminophen Overdose and NAC Dosing". MDCalc. Archived from the original on February 4, 2014. Retrieved February 10, 2014.
- ^ Buckley N, Whyte I, O'Connell D, Dawson A (1999). "Oral or intravenous N-acetylcysteine: which is the treatment of choice for acetaminophen (paracetamol) poisoning?". Journal of Toxicology: Clinical Toxicology. 37 (6): 759–67. doi:10.1081/CLT-100102453. PMID 10584588.
- ^ Baum RA, Woolum JA, Bailey AM, Howell MM, Weant KA, Geraghty L, Mohan S, Webb AN, Su MK, Akpunonu P (July 2021). "Evaluation of Dosing Strategies of N-acetylcysteine for Acetaminophen Toxicity in Patients Greater than 100 Kilograms: Should the Dosage Cap Be Used?". Journal of Medical Toxicology. 17 (3): 241–249. doi:10.1007/s13181-021-00822-x. ISSN 1937-6995. PMC 8206426. PMID 33884558.
- ^ a b c d e Warren G (February 2016). "Trust Wide Intravenous Acetylcysteine for Paracetamol Toxicity in Adults Guideline". Nottingham University Hospitals.
- ^ a b Buckley N, Eddleston M (December 2005). "Paracetamol (acetaminophen) poisoning". Clinical Evidence (14): 1738–44. PMID 16620471.
- ^ Appelboam AV, Dargan PI, Knighton J (November 2002). "Fatal anaphylactoid reaction to N-acetylcysteine: caution in patients with asthma". Emergency Medicine Journal. 19 (6): 594–5. doi:10.1136/emj.19.6.594. PMC 1756296. PMID 12421803.
- ^ Schmidt LE, Dalhoff K (January 2001). "Risk factors in the development of adverse reactions to N-acetylcysteine in patients with paracetamol poisoning". British Journal of Clinical Pharmacology. 51 (1): 87–91. doi:10.1046/j.1365-2125.2001.01305.x. PMC 2014432. PMID 11167669.
- ^ Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT (August 1977). "Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine". Lancet. 2 (8035): 432–4. doi:10.1016/S0140-6736(77)90612-2. PMID 70646. S2CID 44770287.
- ^ Bailey B, McGuigan MA (June 1998). "Management of anaphylactoid reactions to intravenous N-acetylcysteine". Annals of Emergency Medicine. 31 (6): 710–5. doi:10.1016/S0196-0644(98)70229-X. PMID 9624310.
- ^ a b c O'Grady JG, Alexander GJ, Hayllar KM, Williams R (August 1989). "Early indicators of prognosis in fulminant hepatic failure". Gastroenterology. 97 (2): 439–45. doi:10.1016/0016-5085(89)90081-4. PMID 2490426.
- ^ Jaeck D, Boudjema K, Audet M, Chenard-Neu MP, Simeoni U, Meyer C, Nakano H, Wolf P (2002). "Auxiliary partial orthotopic liver transplantation (APOLT) in the treatment of acute liver failure". Journal of Gastroenterology. 37 (Suppl 13): 88–91. doi:10.1007/BF02990107. PMID 12109674. S2CID 21768850.
- ^ Lodge JP, Dasgupta D, Prasad KR, Attia M, Toogood GJ, Davies M, Millson C, Breslin N, Wyatt J, Robinson PJ, Bellamy MC, Snook N, Pollard SG (February 2008). "Emergency subtotal hepatectomy: a new concept for acetaminophen-induced acute liver failure: temporary hepatic support by auxiliary orthotopic liver transplantation enables long-term success". Annals of Surgery. 247 (2): 238–49. doi:10.1097/SLA.0b013e31816401ec. PMID 18216528. S2CID 9408710.
- ^ Bernal W, Donaldson N, Wyncoll D, Wendon J (February 2002). "Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study". Lancet. 359 (9306): 558–63. doi:10.1016/S0140-6736(02)07743-7. PMID 11867109. S2CID 10651412.
- ^ Pereira LM, Langley PG, Hayllar KM, Tredger JM, Williams R (1992). "Coagulation factor V and VIII/V ratio as predictors of outcome in paracetamol induced fulminant hepatic failure: relation to other prognostic indicators". Gut. 33 (1): 98–102. doi:10.1136/gut.33.1.98. PMC 1373872. PMID 1740285.
- ^ Ding GK, Buckley NA (September 2008). "Evidence and consequences of spectrum bias in studies of criteria for liver transplant in paracetamol hepatotoxicity". QJM: Monthly Journal of the Association of Physicians. 101 (9): 723–9. doi:10.1093/qjmed/hcn077. PMID 18606611.
- ^ Sheen C, Dillon J, Bateman D, Simpson K, Macdonald T (2002). "Paracetamol toxicity: epidemiology, prevention and costs to the health-care system". QJM: Monthly Journal of the Association of Physicians. 95 (9): 609–19. doi:10.1093/qjmed/95.9.609. PMID 12205339.
- ^ Hawkins LC, Edwards JN, Dargan PI (2007). "Impact of restricting paracetamol pack sizes on paracetamol poisoning in the United Kingdom: a review of the literature". Drug Safety. 30 (6): 465–79. doi:10.2165/00002018-200730060-00002. PMID 17536874. S2CID 36435353.
- ^ Khashab M, Tector AJ, Kwo PY (March 2007). "Epidemiology of acute liver failure". Current Gastroenterology Reports. 9 (1): 66–73. doi:10.1007/s11894-008-0023-x. PMID 17335680. S2CID 30068892.
- ^ Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM; Acute Liver Failure Study Group. (December 2005). "Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study". Hepatology. 42 (6): 1364–72. doi:10.1002/hep.20948. PMID 16317692. S2CID 24758491.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lee WM (July 2004). "Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure". Hepatology. 40 (1): 6–9. doi:10.1002/hep.20293. PMID 15239078. S2CID 15485538.[dead link]
- ^ Bower WA, Johns M, Margolis HS, Williams IT, Bell BP (November 2007). "Population-based surveillance for acute liver failure". The American Journal of Gastroenterology. 102 (11): 2459–63. doi:10.1111/j.1572-0241.2007.01388.x. PMID 17608778. S2CID 9768605.
External links
[edit]- Gerth J, T. Christian Miller (September 20, 2013). "Use Only as Directed". ProPublica. Retrieved October 12, 2013.


