Vitamin E
| Vitamin E | |
|---|---|
| Drug class | |
 The RRR alpha-tocopherol form of vitamin E | |
| Class identifiers | |
| Use | Vitamin E deficiency, antioxidant |
| ATC code | A11HA03 |
| Biological target | Reactive oxygen species |
| Clinical data | |
| Drugs.com | MedFacts Natural Products |
| External links | |
| MeSH | D014810 |
| Legal status | |
| In Wikidata | |
The term Vitamin E refers to a group of eight molecular-structure related compounds that include four tocopherols and four tocotrienols. The tocopherols function as fat-soluble antioxidants which may help protect cell membranes from reactive oxygen species. Vitamin E is classified as an essential nutrient for humans.[1] [2][3] Various government organizations recommend that adults consume between 3 and 15 mg per day, while a 2016 worldwide review reported a median dietary intake of 6.2 mg per day.[4] Sources rich in vitamin E include seeds, nuts, seed oils, peanut butter, vitamin E-fortified foods and dietary supplements.[3][1] Symptomatic vitamin E deficiency is rare, is usually caused by an underlying problem with digesting dietary fat rather than from a diet low in vitamin E.[5] Deficiency can cause neurological disorders.[1]
Tocopherols and tocotrienols both occur in α (alpha), β (beta), γ (gamma), and δ (delta) forms, as determined by the number and position of methyl groups on the chromanol ring.[1][6] All eight of these vitamers feature a chromane double ring, with a hydroxyl group that can donate a hydrogen atom to reduce free radicals, and a hydrophobic side chain that allows for penetration into biological membranes. Both natural and synthetic tocopherols are subject to oxidation, so dietary supplements are esterified, creating tocopheryl acetate for stability purposes.[3][7]
Population studies have suggested that people who consumed foods with more vitamin E, or who chose on their own to consume a vitamin E dietary supplement, had lower incidence of cardiovascular diseases, cancer, dementia, and other diseases. However, placebo-controlled clinical trials using alpha-tocopherol as a supplement, with daily amounts as high as 2,000 mg per day, could not always replicate these findings.[3] In the United States vitamin E supplement use peaked around 2002, but had declined by more than half by 2006. Declining use was theorized to be due to publications of meta-analyses that showed either no benefits[8][9][10] or actual negative consequences from high-dose vitamin E.[8][11][12]
Vitamin E was discovered in 1922, isolated in 1935, and first synthesized in 1938. Because the vitamin activity was first identified as essential for fertilized eggs to result in live births (in rats), it was given the name "tocopherol" from Greek words meaning birth and to bear or carry. Alpha-tocopherol, either naturally extracted from plant oils or, most commonly, as the synthetic tocopheryl acetate, is sold as a popular dietary supplement, either by itself or incorporated into a multivitamin product, and in oils or lotions for use on skin.
Chemistry
[edit]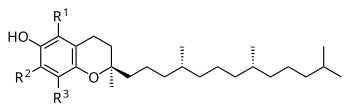

The nutritional content of vitamin E is defined by equivalency to 100% RRR-configuration α-tocopherol activity. The molecules that contribute α-tocopherol activity are four tocopherols and four tocotrienols, within each group of four identified by the prefixes alpha- (α-), beta- (β-), gamma- (γ-), and delta- (δ-). For alpha(α)-tocopherol each of the three "R" sites has a methyl group (CH3) attached. For beta(β)-tocopherol: R1 = methyl group, R2 = H, R3 = methyl group. For gamma(γ)-tocopherol: R1 = H, R2 = methyl group, R3 = methyl group. For delta(δ)-tocopherol: R1 = H, R2 = H, R3 = methyl group. The same configurations exist for the tocotrienols, except that the unsaturated side chain has three carbon-carbon double bonds whereas the tocopherols have a saturated side chain.[13]
Stereoisomers
[edit]In addition to distinguishing tocopherols and tocotrienols by position of methyl groups, the tocopherols have a phytyl tail with three chiral points or centers that can have a right or left orientation. The naturally occurring plant form of alpha-tocopherol is RRR-α-tocopherol, also referred to as d-tocopherol, whereas the synthetic form (all-racemic or all-rac vitamin E, also dl-tocopherol) is equal parts of eight stereoisomers RRR, RRS, RSS, SSS, RSR, SRS, SRR and SSR with progressively decreasing biological equivalency, so that 1.36 mg of dl-tocopherol is considered equivalent to 1.0 mg of d-tocopherol, the natural form. Rephrased, the synthetic has 73.5% of the potency of the natural.[13]
| Form | Structure |
|---|---|
| alpha-Tocopherol | 
|
| beta-Tocopherol | 
|
| gamma-Tocopherol | 
|
| delta-Tocopherol | 
|
| Tocopheryl acetate | 
|
Tocopherols
[edit]Alpha-tocopherol is a lipid-soluble antioxidant functioning within the glutathione peroxidase pathway,[14] and protecting cell membranes from oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction.[3][15] This removes the free radical intermediates and prevents the oxidation reaction from continuing. The oxidized α-tocopheroxyl radicals produced in this process may be recycled back to the active reduced form through reduction by other antioxidants, such as ascorbate, retinol or ubiquinol.[16] Other forms of vitamin E have their own unique properties; for example, γ-tocopherol is a nucleophile that can react with electrophilic mutagens.[6]
Tocotrienols
[edit]The four tocotrienols (alpha, beta, gamma, delta) are similar in structure to the four tocopherols, with the main difference being that the former have hydrophobic side chains with three carbon-carbon double bonds, whereas the tocopherols have saturated side chains. For alpha(α)-tocotrienol each of the three "R" sites has a methyl group (CH3) attached. For beta(β)-tocotrienol: R1 = methyl group, R2 = H, R3 = methyl group. For gamma(γ)-tocotrienol: R1 = H, R2 = methyl group, R3 = methyl group. For delta(δ)-tocotrienol: R1 = H, R2 = H, R3 = methyl group.
Tocotrienols have only a single chiral center, which exists at the 2' chromanol ring carbon, at the point where the isoprenoid tail joins the ring. The other two corresponding centers in the phytyl tail of the corresponding tocopherols do not exist as chiral centers for tocotrienols due to unsaturation (C-C double bonds) at these sites. Tocotrienols extracted from plants are always dextrorotatory stereoisomers, signified as d-tocotrienols. In theory, levorotatory forms of tocotrienols (l-tocotrienols) could exist as well, which would have a 2S rather than 2R configuration at the molecules' single chiral center, but unlike synthetic dl-alpha-tocopherol, the marketed tocotrienol dietary supplements are extracted from palm oil.[17] A number of health benefits of tocotrienols have been proposed, including decreased risk of age-associated cognitive impairment, heart disease and cancer. The evidence is not conclusive.[18][19][20]
Functions
[edit]
Vitamin E may have various roles as a vitamin.[1] Many biological functions have been postulated, including a role as a fat-soluble antioxidant.[1] In this role, vitamin E acts as a radical scavenger, delivering a hydrogen (H) atom to free radicals. At 323 kJ/mol, the O-H bond in tocopherols is about 10% weaker than in most other phenols.[21] This weak bond allows the vitamin to donate a hydrogen atom to the peroxyl radical and other free radicals, minimizing their damaging effect. The thus-generated tocopheryl radical is recycled to tocopherol by a redox reaction with a hydrogen donor, such as vitamin C.[22]
Vitamin E affects gene expression[23] and is an enzyme activity regulator, such as for protein kinase C (PKC) – which plays a role in smooth muscle growth – with vitamin E participating in deactivation of PKC to inhibit smooth muscle growth.[24]
Synthesis
[edit]Biosynthesis
[edit]
Photosynthesizing plants, algae and cyanobacteria synthesize tocochromanols, the chemical family of compounds made up of four tocopherols and four tocotrienols; in a nutrition context this family is referred to as Vitamin E. Biosynthesis starts with formation of the closed-ring part of the molecule as homogentisic acid (HGA). The side chain is attached (saturated for tocopherols, polyunsaturated for tocotrienols). The pathway for both is the same, so that gamma- is created and from that alpha-, or delta- is created and from that the beta- compounds.[25][26] Biosynthesis takes place in the plastids.[26]
As to why plants synthesize tocochromanols, the major reason appears to be for antioxidant activity. Different parts of plants, and different species, are dominated by different tocochromanols. The predominant form in leaves, and hence leafy green vegetables, is α-tocopherol.[25] Location is in chloroplast membranes, in close proximity to the photosynthetic process.[26] The function is to protect against damage from the ultraviolet radiation of sunlight. Under normal growing conditions the presence of α-tocopherol does not appear to be essential, as there are other photo-protective compounds, and plants that through mutations have lost the ability to synthesize α-tocopherol demonstrate normal growth. However, under stressed growing conditions such as drought, elevated temperature or salt-induced oxidative stress, the plants' physiological status is superior if it has the normal synthesis capacity.[27]
Seeds are lipid-rich, to provide energy for germination and early growth. Tocochromanols protect the seed lipids from oxidizing and becoming rancid.[25][26] The presence of tocochromanols extends seed longevity, and promotes successful germination and seedling growth.[27] Gamma-tocopherol dominates in seeds of most plant species, but there are exceptions. For canola, corn and soy bean oils, there is more γ-tocopherol than α-tocopherol, but for safflower, sunflower and olive oils the reverse is true.[25][26][28] Of the commonly used food oils, palm oil is unique in that tocotrienol content is higher than tocopherol content.[28] Seed tocochromanols content is also dependent on environmental stressors. In almonds, for example, drought or elevated temperature increase α-tocopherol and γ-tocopherol content of the nuts. The same article mentions that drought increases the tocopherol content of olives, and heat likewise for soybeans.[29]
Vitamin E biosynthesis occurs in the plastid and goes through two different pathways: the Shikimate pathway and the Methylerythritol Phosphate pathway (MEP pathway).[25] The Shikimate pathway generates the chromanol ring from the Homogentisic Acid (HGA) and the MEP pathway produces the hydrophobic tail which differs between tocopherol and tocotrienol. The synthesis of the specific tail is dependent on which molecule it originates from. In a tocopherol, its prenyl tail emerges from the geranylgeranyl diphosphate (GGDP) group, while the phytyl tail of a tocotrienol stems from a phytyl diphosphate.[25]
Industrial synthesis
[edit]The synthetic product is all-rac-alpha-tocopherol,[30] also referred to as dl-alpha tocopherol. It consists of eight stereoisomers (RRR, RRS, RSS, RSR, SRR, SSR, SRS and SSS) in equal quantities. "It is synthesized from a mixture of toluene and 2,3,5-trimethyl-hydroquinone that reacts with isophytol to all-rac-alpha-tocopherol, using iron in the presence of hydrogen chloride gas as catalyst. The reaction mixture obtained is filtered and extracted with aqueous caustic soda. Toluene is removed by evaporation and the residue (all rac-alpha-tocopherol) is purified by vacuum distillation."[30] The natural alpha tocopherol extracted from plants is RRR-alpha tocopherol, referred to as d-alpha-tocopherol.[31] The synthetic has 73.5% of the potency of the natural.[32] Manufacturers of dietary supplements and fortified foods for humans or domesticated animals convert the phenol form of the vitamin to an ester using either acetic acid or succinic acid because the esters are more chemically stable, providing for a longer shelf-life.[3][33]
Deficiency
[edit]A worldwide summary of more than one hundred human studies reported a median of 22.1 μmol/L for serum α-tocopherol, and defined α-tocopherol deficiency as less than 12 μmol/L. It cited a recommendation that serum α-tocopherol concentration be ≥30 μmol/L to optimize health benefits.[4] In contrast, the U.S. Dietary Reference Intake text for vitamin E concluded that a plasma concentration of 12 μmol/L was sufficient to achieve normal ex vivo hydrogen peroxide-induced hemolysis.[5] A 2014 review defined less than 9 μmol/L as deficient, 9-12 μmol/L as marginal, and greater than 12 μmol/L as adequate.[34]
Regardless of which definition is used, vitamin E deficiency is rare in humans, occurring as a consequence of abnormalities in dietary fat absorption or metabolism rather than from a diet low in vitamin E.[5] Cystic fibrosis and other fat malabsorption conditions can result in low serum vitamin E.[1] One example of a genetic abnormality in metabolism is mutations of genes coding for alpha-tocopherol transfer protein (α-TTP). Humans with this genetic defect exhibit a progressive neurodegenerative disorder known as ataxia with vitamin E deficiency (AVED) despite consuming normal amounts of vitamin E. Large amounts of alpha-tocopherol as a dietary supplement are needed to compensate for the lack of α-TTP.[35][36]
Bariatric surgery as a treatment for obesity can lead to vitamin deficiencies. Long-term follow-up reported a 16.5% prevalence of vitamin E deficiency.[37] There are guidelines for multivitamin supplementation, but adherence rates are reported to be less than 20%.[38]
Vitamin E deficiency due to either malabsorption or metabolic anomaly can cause nerve problems due to poor conduction of electrical impulses along nerves due to changes in nerve membrane structure and function. In addition to ataxia, vitamin E deficiency can cause peripheral neuropathy, myopathies, retinopathy, and impairment of immune responses.[5][1]
Drug interactions
[edit]The amounts of alpha-tocopherol, other tocopherols and tocotrienols that are components of dietary vitamin E, when consumed from foods, do not appear to cause any interactions with drugs. Consumption of alpha-tocopherol as a dietary supplement in amounts in excess of 300 mg/day may lead to interactions with aspirin, warfarin and cyclosporine A in ways that alter function.[1][39] For aspirin and warfarin, high amounts of vitamin E may potentiate anti-blood clotting action.[1][39] In multiple clinical trials, vitamin E lowered blood concentration of the immunosuppressant medication, cyclosporine A.[39] The US National Institutes of Health, Office of Dietary Supplements, raises a concern that co-administration of vitamin E could counter the mechanisms of anti-cancer radiation therapy and some types of chemotherapy, and so advises against its use in these patient populations. The references it cited reported instances of reduced treatment adverse effects, but also poorer cancer survival, raising the possibility of tumor protection from the intended oxidative damage by the treatments.[1]
Dietary recommendations
[edit]| US vitamin E recommendations (mg per day)[5] | |
|---|---|
| AI (children ages 0–6 months) | 4 |
| AI (children ages 7–12 months) | 5 |
| RDA (children ages 1–3 years) | 6 |
| RDA (children ages 4–8 years) | 7 |
| RDA (children ages 9–13 years) | 11 |
| RDA (children ages 14–18 years) | 15 |
| RDA (adults ages 19+) | 15 |
| RDA (pregnancy) | 15 |
| RDA (lactation) | 19 |
| UL (adults) | 1,000 |
The U.S. National Academy of Medicine updated estimated average requirements (EARs) and recommended dietary allowances (RDAs) for vitamin E in 2000. RDAs are higher than EARs so as to identify amounts that will cover people with higher than average requirements. Adequate intakes (AIs) are identified when there is not sufficient information to set EARs and RDAs. The EAR for vitamin E for women and men ages 14 and up is 12 mg/day. The RDA is 15 mg/day.[5] As for safety, tolerable upper intake levels ("upper limits" or ULs) are set for vitamins and minerals when evidence is sufficient. Hemorrhagic effects in rats were selected as the critical endpoint to calculate the upper limit via starting with the lowest-observed-adverse-effect-level. The result was a human upper limit set at 1000 mg/day.[5] Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes.[5]
The European Food Safety Authority (EFSA) refers to the collective set of information as dietary reference values, with population reference intakes (PRIs) instead of RDAs, and average requirements instead of EARs. AIs and ULs are defined the same as in the United States. For women and men ages 10 and older, the PRIs are set at 11 and 13 mg/day, respectively. PRI for pregnancy is 11 mg/day, for lactation 11 mg/day. For children ages 1–9 years the PRIs increase with age from 6 to 9 mg/day.[40] The EFSA used an effect on blood clotting as a safety-critical effect. It identified that no adverse effects were observed in a human trial as 540 mg/day, used an uncertainty factor of 2 to derive an upper limit of half of that, then rounded to 300 mg/day.[41]
The People's Republic of China publishes dietary guidelines without specifics for individual vitamins or minerals.[42] The United Kingdom recommends 4 mg/day for adult men and 3 mg/day for adult women.[43] The Japan National Institute of Health and Nutrition set adult AIs at 6.5 mg/day (females) and 7.0 mg/day (males), and 650–700 mg/day (females), and 750–900 mg/day (males) for upper limits (amounts depending on age).[44] India recommends an intake of 8–10 mg/day and does not set an upper limit.[45] The World Health Organization recommends that adults consume 10 mg/day.[4]
Consumption tends to be below these recommendations. A worldwide summary reported a median dietary intake of 6.2 mg/d for alpha-tocopherol.[4]
Food labeling
[edit]For U.S. food and dietary supplement labeling purposes, the amount in a serving is expressed as a percent of daily value. For vitamin E labeling purposes 100% of the daily value was 30 international units, but as of 27 May 2016, it was revised to 15 mg to bring it into agreement with the RDA.[46] A table of the old and new adult daily values is provided at Reference Daily Intake.
European Union regulations require that labels declare energy, protein, fat, saturated fat, carbohydrates, sugars, and salt. Voluntary nutrients may be shown if present in significant amounts. Instead of daily values, amounts are shown as percent of reference intakes (RIs). For vitamin E, 100% RI was set at 12 mg in 2011.[47]
The international unit measurement was used by the United States in 1968–2016. 1 IU is the biological equivalent of about 0.667 mg d (RRR)-alpha-tocopherol (2/3 mg exactly), or of 0.90 mg of dl-alpha-tocopherol, corresponding to the then-measured relative potency of stereoisomers. In May 2016, the measurements have been revised, such that 1 mg of "Vitamin E" is 1 mg of d-alpha-tocopherol or 2 mg of dl-alpha-tocopherol.[48] The change was originally started in 2000, when forms of Vitamin E other than alpha-tocopherol was dropped from dietary calculations by the IOM. The UL amount disregards any conversion.[49] The EFSA has never used an IU unit, and their measurement only considers RRR-alpha-tocopherol.[50]
Sources
[edit]Of the different forms of vitamin E, gamma-tocopherol (γ-tocopherol) is the most common form found in the North American diet, but alpha-tocopherol (α-tocopherol) is the most biologically active.[3][51]
The U.S. Department of Agriculture (USDA), Agricultural Research Services, maintains a food composition database. The last major revision was Release 28, September 2015. In addition to the naturally occurring sources shown in the table,[52] certain ready-to-eat cereals, infant formulas, liquid nutrition products and other foods are fortified with alpha-tocopherol.[52]
| Plant source[52] | Amount (mg / 100 g) |
|---|---|
| Wheat germ oil | 150 |
| Hazelnut oil | 47 |
| Canola/rapeseed oil | 44 |
| Sunflower oil | 41.1 |
| Almond oil | 39.2 |
| Safflower oil | 34.1 |
| Grapeseed oil | 28.8 |
| Sunflower seed kernels | 26.1 |
| Almonds | 25.6 |
| Plant source[52] | Amount (mg / 100 g) |
|---|---|
| Palm oil | 15.9 |
| Peanut oil | 15.7 |
| Margarine, tub | 15.4 |
| Hazelnuts | 15.3 |
| Corn oil | 14.8 |
| Olive oil | 14.3 |
| Soybean oil | 12.1 |
| Pine nuts | 9.3 |
| Peanut butter | 9.0 |
| Plant source[52] | Amount (mg / 100 g) |
|---|---|
| Pistachio nuts | 2.8 |
| Avocados | 2.6 |
| Spinach, raw | 2.0 |
| Asparagus | 1.5 |
| Broccoli | 1.4 |
| Cashew nuts | 0.9 |
| Bread | 0.2-0.3 |
| Rice, brown | 0.2 |
| Potato, Pasta | <0.1 |
| Animal source[52] | Amount (mg / 100 g) |
|---|---|
| Fish | 1.0-2.8 |
| Oysters | 1.7 |
| Butter | 1.6 |
| Cheese | 0.6-0.7 |
| Eggs | 1.1 |
| Chicken | 0.3 |
| Beef, Pork | 0.1 |
| Milk, whole | 0.1 |
| Milk, skim | 0.01 |
Tocotrienols occur in some foods sources, the richest being palm oil, but to a lesser extent rice bran oil, barley, oats, and certain seeds, nuts and grains, and the oils derived from them.[53][54]
Supplements
[edit]
Vitamin E is fat soluble, so dietary supplement products are usually in the form of the vitamin, esterified with acetic acid to generate tocopheryl acetate, and dissolved in vegetable oil in a softgel capsule.[3] For alpha-tocopherol, amounts range from 100 to 1000 IU per serving. Smaller amounts are incorporated into multi-vitamin/mineral tablets. Gamma-tocopherol and tocotrienol supplements are also available from dietary supplement companies. The latter are extracts from palm oil.[17]
Fortification
[edit]The World Health Organization does not have any recommendations for food fortification with vitamin E.[55] The Food Fortification Initiative does not list any countries that have mandatory or voluntary programs for vitamin E.[56] Infant formulas have alpha-tocopherol as an ingredient. In some countries, certain brands of ready-to-eat cereals, liquid nutrition products and other foods have alpha-tocopherol as an added ingredient.[52]
Non-nutrient food additives
[edit]Various forms of vitamin E are common food additive in oily food, used to deter rancidity caused by peroxidation. Those with an E number include:[57]
- E306 Tocopherol-rich extract (mixed, natural, can include tocotrienol)
- E307 Alpha-tocopherol (synthetic)
- E308 Gamma-tocopherol (synthetic)
- E309 Delta-tocopherol (synthetic)
These E numbers include all racemic forms and acetate esters thereof.[57] Commonly found on food labels in Europe and some other countries, their safety assessment and approval are the responsibility of the European Food Safety Authority.[58]
Absorption, metabolism, excretion
[edit]Tocotrienols and tocopherols, the latter including the stereoisomers of synthetic alpha-tocopherol, are absorbed from the intestinal lumen, incorporated into chylomicrons, and secreted into the portal vein, leading to the liver. Absorption efficiency is estimated at 51% to 86%,[5] and that applies to all of the vitamin E family – there is no discrimination among the vitamin E vitamers during absorption. Bile is necessary for chylomicron formation, so disease conditions such as cystic fibrosis result in biliary insufficiency and vitamin E malabsorption.[2] When consumed as an alpha-tocopheryl acetate dietary supplement, absorption is promoted when consumed with a fat-containing meal.[2] Unabsorbed vitamin E is excreted via feces. Additionally, vitamin E is excreted by the liver via bile into the intestinal lumen, where it will either be reabsorbed or excreted via feces, and all of the vitamin E vitamers are metabolized and then excreted via urine.[5][13]
Upon reaching the liver, RRR-alpha-tocopherol is preferentially taken up by alpha-tocopherol transfer protein (α-TTP). All other forms are degraded to 2'-carboxethyl-6-hydroxychromane (CEHC), a process that involves truncating the phytic tail of the molecule, then either sulfated or glycuronidated. This renders the molecules water-soluble and leads to excretion via urine. Alpha-tocopherol is also degraded by the same process, to 2,5,7,8-tetramethyl-2-(2'-carboxyethyl)-6-hydroxychromane (α-CEHC), but more slowly because it is partially protected by α-TTP. Large intakes of α-tocopherol result in increased urinary α-CEHC, so this appears to be a means of disposing of excess vitamin E.[5][13]
Alpha-tocopherol transfer protein is coded by the TTPA gene on chromosome 8. The binding site for RRR-α-tocopherol is a hydrophobic pocket with a lower affinity for beta-, gamma-, or delta-tocopherols, or for the stereoisomers with an S configuration at the chiral 2 site. Tocotrienols are also a poor fit because the double bonds in the phytic tail create a rigid configuration that is a mismatch with the α-TTP pocket.[13] A rare genetic defect of the TTPA gene results in people exhibiting a progressive neurodegenerative disorder known as ataxia with vitamin E deficiency (AVED) despite consuming normal amounts of vitamin E. Large amounts of alpha-tocopherol as a dietary supplement are needed to compensate for the lack of α-TTP.[35] The role of α-TTP is to move α-tocopherol to the plasma membrane of hepatocytes (liver cells), where it can be incorporated into newly created very low density lipoprotein (VLDL) molecules. These convey α-tocopherol to cells in the rest of the body. As an example of a result of the preferential treatment, the US diet delivers approximately 70 mg/d of γ-tocopherol and plasma concentrations are on the order of 2–5 μmol/L; meanwhile, dietary α-tocopherol is about 7 mg/d but plasma concentrations are in the range of 11–37 μmol/L.[13]
Affinity of α-TTP for vitamin E vitamers[13]
| Vitamin E compound | Affinity |
|---|---|
| RRR-alpha-tocopherol | 100% |
| beta-tocopherol | 38% |
| gamma-tocopherol | 9% |
| delta-tocopherol | 2% |
| SSR-alpha-tocopherol | 11% |
| alpha-tocotrienol | 12% |
Medical applications
[edit]Vitamin E has been suggested as a supplement for helping many health conditions, mostly due to its antioxidant activity and potential to protect cells from oxidative damage. In the US, the vitamin is widely available as an over-the-counter supplement, however, medical evidence supporting its effectiveness and safety for treating or preventing a variety of health conditions is mixed. Vitamin E can also interact with some medications and other supplements.[1] Vitamin E has been studied as a treatment for skin health and skin ageing, immune function,[59] and managing conditions like cardiovascular disease[60] or Alzheimer's disease (AD),[61] or certain types of cancer.[60] Most studies have found limited or inconclusive benefits and the potential for some risks. It is most often recommended to obtain vitamin E through a balanced diet because high-dose supplementation may have health risks.[1] There is evidence that the sale of dietary supplement vitamin E has decreased by up to 33% following a report showing little or no effect of vitamin E in preventing cancer or cardiovascular disease.[11]
All-cause mortality
[edit]Two meta-analyses concluded that as a dietary supplement, vitamin E neither improved nor impaired all-cause mortality.[9][10] A meta-analysis of long-term clinical trials reported a non-significant 2% increase in all-cause mortality when alpha-tocopherol was the only supplement used. The same journal article reported a statistically significant 3% increase for results when alpha-tocopherol was used in combination with other nutrients (vitamin A, vitamin C, beta-carotene, selenium).[12]
Age-related macular degeneration
[edit]A Cochrane review concluded that there were no changes seen for risk of developing age-related macular degeneration (AMD) from long-term vitamin E supplementation and that supplementation may slightly increase the chances of developing late AMD.[62]
Cognitive impairment and Alzheimer's disease
[edit]Two meta-analyses reported lower vitamin E blood levels in AD people compared to healthy, age-matched people.[63][64] However, a review of vitamin E supplementation trials concluded that there was insufficient evidence to state that supplementation reduced the risk of developing AD or slowed the progression of AD.[61]
Cancer
[edit]In a 2022 update of an earlier report, the United States Preventive Services Task Force recommended against the use of vitamin E supplements for the prevention of cardiovascular disease or cancer, concluding there was insufficient evidence to assess the balance of benefits and harms, yet also concluding with moderate certainty that there is no net benefit of supplementation.[60]
As for literature on different types of cancer, an inverse relationship between dietary vitamin E and kidney cancer and bladder cancer is seen in observational studies.[65][66] A large clinical trial reported no difference in bladder cancer cases between treatment and placebo.[67]
An inverse relationship between dietary vitamin E and lung cancer was reported in observational studies,[68] but a large clinical trial in male tobacco smokers reported no impact on lung cancer between treatment and placebo,[69] and a trial which tracked people who chose to consume a vitamin E dietary supplement reported an increased risk of lung cancer for those consuming more than 215 mg/day.[70]
For prostate cancer, there are also conflicting results. A meta-analysis based on serum alpha-tocopherol content reported an inverse correlation in relative risk,[71] but a second meta-analysis of observational studies reported no such relationship.[72] A large clinical trial with male tobacco smokers and reported a 32% decrease in the incidence of prostate cancer,[73] but the SELECT trial of selenium or vitamin E for prostate cancer enrolled men ages 55 or older and reported relative risk 17% higher for the vitamin group.[74]
For colorectal cancer, a systematic review of randomized clinical trials and the large SELECT trial reported no statistically significant change in relative risk.[75][76] The Women's Health Study reported no significant differences for incidences of all types of cancer, cancer deaths, or specifically for breast, lung or colon cancers.[77]
Potential confounding factors are the form of vitamin E used in prospective studies and the amounts. Synthetic, racemic mixtures of vitamin E isomers are not bioequivalent to natural, non-racemic mixtures, yet are widely used in clinical trials and as dietary supplement ingredients.[78] One review reported a modest increase in cancer risk with vitamin E supplementation while stating that more than 90% of the cited clinical trials used the synthetic, racemic form dl-alpha-tocopherol.[70]
Cancer health claims
[edit]The U.S. Food and Drug Administration initiated a process of reviewing and approving food and dietary supplement health claims in 1993. Reviews of petitions results in proposed claims being rejected or approved. If approved, specific wording is allowed on package labels. In 1999, a second process for claims review was created. If there is not a scientific consensus on the totality of the evidence, a Qualified Health Claim (QHC) may be established. The FDA does not "approve" qualified health claim petitions. Instead, it issues a Letter of Enforcement Discretion that includes very specific claim language and the restrictions on using that wording.[79] The first QHCs relevant to vitamin E were issued in 2003: "Some scientific evidence suggests that consumption of antioxidant vitamins may reduce the risk of certain forms of cancer." In 2009, the claims became more specific, allowing that vitamin E might reduce the risk of renal, bladder and colorectal cancers, but with required mention that the evidence was deemed weak and the claimed benefits highly unlikely. A petition to add brain, cervical, gastric and lung cancers was rejected. A further revision, May 2012, allowed that vitamin E may reduce risk of renal, bladder and colorectal cancers, with a more concise qualifier sentence added: "FDA has concluded that there is very little scientific evidence for this claim." Any company product label making the cancer claims has to include a qualifier sentence.[80]
Cataracts
[edit]A review measured serum tocopherol and reported higher serum concentration was associated with a 23% reduction in relative risk of age-related cataracts (ARC), with the effect due to differences in nuclear cataract rather than cortical or posterior subcapsular cataract.[81] In contrast, meta-analyses reporting on clinical trials of alpha-tocopherol supplementation reported no statistically significant change to risk of ARC compared to placebo.[81][82]
Cardiovascular diseases
[edit]In a 2022 update of an earlier report, the United States Preventive Services Task Force recommended against the use of vitamin E supplements for the prevention of cardiovascular disease or cancer, concluding there was insufficient evidence to assess the balance of benefits and harms, yet also concluding with moderate certainty that there is no net benefit of supplementation.[60]
Research on the effects of vitamin E on cardiovascular disease has produced conflicting results. In theory, oxidative modification of LDL-cholesterol promotes blockages in coronary arteries that lead to atherosclerosis and heart attacks, so vitamin E functioning as an antioxidant would reduce oxidized cholesterol and lower risk of cardiovascular disease. Vitamin E status has also been implicated in the maintenance of normal endothelial cell function of cells lining the inner surface of arteries, anti-inflammatory activity and inhibition of platelet adhesion and aggregation.[83] An inverse relation has been observed between coronary heart disease and the consumption of foods high in vitamin E, and also higher serum concentration of alpha-tocopherol.[83][84] The problem with observational studies is that these cannot confirm a relation between the lower risk of coronary heart disease and vitamin E consumption diets higher in vitamin E may also be higher in other, unidentified components that promote heart health, or lower in diet components detrimental to heart health, or people choosing such diets may be making other healthy lifestyle choices.[83]
A meta-analysis of randomized clinical trials (RCTs) reported that when consumed without any other antioxidant nutrient, the relative risk of heart attack was reduced by 18%.[85] However, two large trials that were incorporated into the meta-analysis either did not show any benefit for heart attack, stroke, coronary mortality or all-cause mortality,[86] or else a higher risk of heart failure in the alpha-tocopherol group.[87]
Vitamin E supplementation does not reduce the incidence of ischemic or hemorrhagic stroke.[88][89] However, supplementation of vitamin E with other antioxidants reduced risk of ischemic stroke by 9% while increased the risk for hemorrhagic stroke by 22%.[89]
Denial of cardiovascular health claims
[edit]In 2001, the U.S. Food and Drug Administration rejected proposed health claims for vitamin E and cardiovascular health.[90] The U.S. National Institutes of Health reviewed literature published up to 2008 and concluded "In general, clinical trials have not provided evidence that routine use of vitamin E supplements prevents cardiovascular disease or reduces its morbidity and mortality."[1] The European Food Safety Authority (EFSA) reviews proposed health claims for the European Union countries. In 2010, the EFSA reviewed and rejected claims that a cause and effect relationship has been established between the dietary intake of vitamin E and maintenance of normal cardiac function or of normal blood circulation.[91]
Nonalcoholic fatty liver disease
[edit]Supplemental vitamin E significantly reduced elevated liver enzymes, steatosis, inflammation and fibrosis, suggesting that the vitamin may be useful for treatment of nonalcoholic fatty liver disease (NAFLD) and the more extreme subset known as nonalcoholic steatohepatitis (NASH) in adults,[92][93][94] but not in children.[95][96]
Exercise recovery
[edit]In healthy adults, after exercise, vitamin E was shown to not have any benefits for post-exercise recovery, as measured by muscle soreness and muscle strength, or measured by indicators for inflammation or muscle damage, such as interleukin-6 and creatine kinase.[97]
Parkinson's disease
[edit]For Parkinson's disease, there is an observed inverse correlation seen with dietary vitamin E, but no confirming evidence from placebo-controlled clinical trials.[98][99]
Pregnancy
[edit]Supplementation with a combination of vitamins E and C during pregnancy is not recommended by the World Health Organization.[100] A Cochrane review concluded there was no support for the combination reducing risk of stillbirth, neonatal death, preterm birth, preeclampsia or any other maternal or infant outcomes, either in healthy women or those considered at risk for pregnancy complications.[101]
Topical applications
[edit]There is widespread use of tocopheryl acetate in some skincare and wound-treatment products as a topical medication, with claims for improved wound healing and reduced scar tissue,[102] but reviews have repeatedly concluded that there is insufficient evidence to support these claims.[103][104] There are also reports of allergic contact dermatitis from use of vitamin-E derivatives such as tocopheryl linoleate and tocopherol acetate in skin care products.[105]
Vaping-associated lung injury
[edit]The US Centers for Disease Control and Prevention (CDC) stated in February 2020 that previous research suggested inhaled vitamin E acetate (α-tocopheryl acetate) may interfere with normal lung functioning.[106] In September 2019, the US Food and Drug Administration had announced that vape liquids linked to recent vaping related lung disease outbreak in the United States, tested positive for vitamin E acetate[107] which had been used as a thickening agent by illicit THC vape cartridge manufacturers.[108] By November 2019, the CDC had identified vitamin E acetate as a very strong culprit of concern in the vaping-related illnesses, but has not ruled out other chemicals or toxicants as possible causes. These findings were based on fluid samples from the lungs of people with vaping-associated pulmonary injury.[109][110] Pyrolysis of vitamin E acetate produces exceptionally toxic ketene gas, along with carcinogenic alkenes and benzene.[111]
History
[edit]Vitamin E was discovered in 1922 by Herbert McLean Evans and Katharine Scott Bishop[112] and first isolated in a pure form by Evans and Gladys Anderson Emerson in 1935 at the University of California, Berkeley.[113] Because the vitamin activity was first identified as a dietary fertility factor in rats, it was given the name "tocopherol" from the Greek words "τόκος" [tókos, birth], and "φέρειν", [phérein, to bear or carry] meaning in sum "to carry a pregnancy," with the ending "-ol" signifying its status as a chemical alcohol. George M. Calhoun, Professor of Greek at the University of California, was credited with helping with the naming process.[114] Erhard Fernholz elucidated its structure in 1938 and shortly afterward the same year, Paul Karrer and his team first synthesized it.[115]
Nearly 50 years after the discovery of vitamin E, an editorial in the Journal of the American Medical Association titled "Vitamin in search of a disease" read in part "...research revealed many of the vitamin's secrets, but no certain therapeutic use and no definite deficiency disease in man." The animal discovery experiments had been a requirement for successful pregnancy, but no benefits were observed for women prone to miscarriage. Evidence for vascular health was characterized as unconvincing. The editorial closed with mention of some preliminary human evidence for protection against hemolytic anemia in young children.[116]
A role for vitamin E in coronary heart disease was first proposed in 1946 by Evan Shute and colleagues.[117][118] More cardiovascular work from the same research group followed,[119] including a proposal that megadoses of vitamin E could slow down and even reverse the development of atherosclerosis.[120] Subsequent research showed no association between vitamin E supplementation and cardiovascular events such as nonfatal stroke or myocardial infarction, or cardiovascular mortality.[121]
There is a long history of belief that topical application of vitamin E containing oil benefits burn and wound healing.[102] This belief persists even though scientific reviews refuted this claim.[103][104]
The role of vitamin E in infant nutrition has a long research history. From 1949 onward there were trials with premature infants suggesting that oral alpha-tocopherol was protective against edema, intracranial hemorrhage, hemolytic anemia and retrolental fibroplasia.[122] A more recent review concluded that vitamin E supplementation in preterm infants reduced the risk of intracranial hemorrhage and retinopathy, but noted an increased risk of sepsis.[123]
References
[edit]- ^ a b c d e f g h i j k l m n "Vitamin E Fact Sheet for Health Profesionals". Office of Dietary Supplements, U.S. National Institutes of Health. 12 July 2019. Retrieved 20 October 2024.
- ^ a b c Traber MG, Bruno RS (2020). "Vitamin E". In Marriott BP, Birt DF, Stallings VA, Yates AA (eds.). Present knowledge in nutrition, eleventh edition. London, United Kingdom: Academic Press (Elsevier). pp. 115–36. ISBN 978-0-323-66162-1.
- ^ a b c d e f g h "Vitamin E". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. October 2015. Retrieved 3 August 2019.
- ^ a b c d Péter S, Friedel A, Roos FF, Wyss A, Eggersdorfer M, Hoffmann K, et al. (December 2015). "A systematic review of global alpha-tocopherol status as assessed by nutritional intake levels and blood serum concentrations". International Journal for Vitamin and Nutrition Research. 85 (5–6): 261–81. doi:10.1024/0300-9831/a000281. PMID 27414419.
- ^ a b c d e f g h i j k Institute of Medicine (2000). "Vitamin E". Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: The National Academies Press. pp. 186–283. doi:10.17226/9810. ISBN 978-0-309-06935-9. PMID 25077263.
- ^ a b Brigelius-Flohé R, Traber MG (July 1999). "Vitamin E: function and metabolism". FASEB Journal. 13 (10): 1145–55. doi:10.1096/fasebj.13.10.1145. PMID 10385606. S2CID 7031925.
- ^ Braunstein MH (March 2006). Focus on vitamin E research. Nova Science Publishers. p. vii. ISBN 978-1-59454-971-7.
- ^ a b Kim HJ, Giovannucci E, Rosner B, Willett WC, Cho E (March 2014). "Longitudinal and secular trends in dietary supplement use: Nurses' Health Study and Health Professionals Follow-Up Study, 1986–2006". Journal of the Academy of Nutrition and Dietetics. 114 (3): 436–43. doi:10.1016/j.jand.2013.07.039. PMC 3944223. PMID 24119503.
- ^ a b Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ (July 2011). "Vitamin E and all-cause mortality: a meta-analysis". Current Aging Science. 4 (2): 158–70. doi:10.2174/1874609811104020158. PMC 4030744. PMID 21235492.
- ^ a b Curtis AJ, Bullen M, Piccenna L, McNeil JJ (December 2014). "Vitamin E supplementation and mortality in healthy people: a meta-analysis of randomised controlled trials". Cardiovasc Drugs Ther. 28 (6): 563–73. doi:10.1007/s10557-014-6560-7. PMID 25398301. S2CID 23820017.
- ^ a b Tilburt JC, Emanuel EJ, Miller FG (September 2008). "Does the evidence make a difference in consumer behavior? Sales of supplements before and after publication of negative research results". Journal of General Internal Medicine. 23 (9): 1495–8. doi:10.1007/s11606-008-0704-z. PMC 2518024. PMID 18618194.
- ^ a b Bjelakovic G, Nikolova D, Gluud C (2013). "Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm?". PLOS ONE. 8 (9): e74558. Bibcode:2013PLoSO...874558B. doi:10.1371/journal.pone.0074558. PMC 3765487. PMID 24040282.
- ^ a b c d e f g Manolescu B, Atanasiu V, Cercasov C, Stoian I, Oprea E, Buşu C (October–December 2008). "So many options but one choice: the human body prefers alpha-tocopherol. A matter of stereochemistry". Journal of Medicine and Life. 1 (4): 376–82. PMC 5654212. PMID 20108516.
- ^ Wefers H, Sies H (June 1988). "The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E". European Journal of Biochemistry. 174 (2): 353–7. doi:10.1111/j.1432-1033.1988.tb14105.x. PMID 3383850.
- ^ Traber MG, Atkinson J (July 2007). "Vitamin E, antioxidant and nothing more". Free Radical Biology & Medicine. 43 (1): 4–15. doi:10.1016/j.freeradbiomed.2007.03.024. PMC 2040110. PMID 17561088.
- ^ Wang X, Quinn PJ (July 1999). "Vitamin E and its function in membranes". Progress in Lipid Research. 38 (4): 309–36. doi:10.1016/S0163-7827(99)00008-9. PMID 10793887.
- ^ a b Ahsan H, Ahad A, Siddiqui WA (April 2015). "A review of characterization of tocotrienols from plant oils and foods". J Chem Biol. 8 (2): 45–59. doi:10.1007/s12154-014-0127-8. PMC 4392014. PMID 25870713.
- ^ Meganathan P, Fu JY (October 2016). "Biological properties of tocotrienols: evidence in human studies". International Journal of Molecular Sciences. 17 (11): E1682. doi:10.3390/ijms17111682. PMC 5133770. PMID 27792171.
- ^ Georgousopoulou EN, Panagiotakos DB, Mellor DD, Naumovski N (January 2017). "Tocotrienols, health and ageing: A systematic review" (PDF). Maturitas. 95: 55–60. doi:10.1016/j.maturitas.2016.11.003. PMID 27889054.
- ^ Prasad K (2011). "Tocotrienols and cardiovascular health". Current Pharmaceutical Design. 17 (21): 2147–54. doi:10.2174/138161211796957418. PMID 21774782.
- ^ "Handbook of chemistry and physics 102nd edition". CRC Press. Archived from the original on 24 April 2021. Retrieved 12 December 2022.
- ^ Traber MG, Stevens JF (September 2011). "Vitamins C and E: beneficial effects from a mechanistic perspective". Free Radical Biology & Medicine. 51 (5): 1000–13. doi:10.1016/j.freeradbiomed.2011.05.017. PMC 3156342. PMID 21664268.
- ^ Azzi A (June 2018). "Many tocopherols, one vitamin E". Molecular Aspects of Medicine. 61: 92–103. doi:10.1016/j.mam.2017.06.004. PMID 28624327. S2CID 36083439.
- ^ Schneider C (January 2005). "Chemistry and biology of vitamin E". Molecular Nutrition & Food Research. 49 (1): 7–30. doi:10.1002/mnfr.200400049. PMID 15580660.
- ^ a b c d e f Mène-Saffrané L (January 2018). "Vitamin E biosynthesis and its regulation in plants". Antioxidants. 7 (1): 2. doi:10.3390/antiox7010002. PMC 5789312. PMID 29295607.
- ^ a b c d e Fritsche S, Wang X, Jung C (December 2017). "Recent advances in our understanding of tocopherol biosynthesis in plants: an overview of key genes, functions, and breeding of vitamin E improved crops". Antioxidants. 6 (4): 99. doi:10.3390/antiox6040099. PMC 5745509. PMID 29194404.
- ^ a b Falk J, Munné-Bosch S (June 2010). "Tocochromanol functions in plants: antioxidation and beyond". Journal of Experimental Botany. 61 (6): 1549–66. doi:10.1093/jxb/erq030. PMID 20385544.
- ^ a b Shahidi F, de Camargo AC (October 2016). "Tocopherols and tocotrienols in common and emerging dietary sources: occurrence, applications, and health benefits". International Journal of Molecular Sciences. 17 (10): 1745. doi:10.3390/ijms17101745. PMC 5085773. PMID 27775605.
- ^ Kodad O, Socias i Company R, Alonso JM (January 2018). "Genotypic and environmental effects on tocopherol content in almond". Antioxidants. 7 (1): 6. doi:10.3390/antiox7010006. PMC 5789316. PMID 29303980.
- ^ a b "Scientific opinion on the safety and efficacy of synthetic alpha-tocopherol for all animal species". EFSA Journal. 10 (7): 2784. July 2012. doi:10.2903/j.efsa.2012.2784.
- ^ Brigelius-Flohé R, Traber MG (July 1999). "Vitamin E: function and metabolism". FASEB Journal. 13 (10): 1145–1155. doi:10.1096/fasebj.13.10.1145. PMID 10385606.
- ^ Traber MG (1999). "Utilization of vitamin E". BioFactors. 10 (2–3): 115–120. doi:10.1002/biof.5520100205. PMID 10609871. S2CID 26970237.
- ^ Zou Z, Dai L, Liu D, Du W (June 2021). "Research progress in enzymatic synthesis of vitamin E ester derivatives". Catalysts. 11 (6): 739. doi:10.3390/catal11060739.
- ^ Traber MG (September 2014). "Vitamin E inadequacy in humans: causes and consequences". Advances in Nutrition. 5 (5): 503–14. doi:10.3945/an.114.006254. PMC 4188222. PMID 25469382.
- ^ a b Christopher Min K (2007). "Structure and Function of α-Tocopherol Transfer Protein: Implications for Vitamin e Metabolism and AVED". Structure and function of alpha-tocopherol transfer protein: implications for vitamin E metabolism and AVED. Vitamins & Hormones. Vol. 76. pp. 23–43. doi:10.1016/S0083-6729(07)76002-8. ISBN 978-0-12-373592-8. PMID 17628170.
- ^ Niki E, Traber MG (November 2012). "A history of vitamin E". Annals of Nutrition & Metabolism. 61 (3): 207–12. doi:10.1159/000343106. PMID 23183290. S2CID 25667777.
- ^ Chen L, Chen Y, Yu X, Liang S, Guan Y, Yang J, et al. (July 2024). "Long-term prevalence of vitamin deficiencies after bariatric surgery: a meta-analysis". Langenbecks Arch Surg. 409 (1): 226. doi:10.1007/s00423-024-03422-9. PMID 39030449.
- ^ Ha J, Kwon Y, Kwon JW, Kim D, Park SH, Hwang J, et al. (July 2021). "Micronutrient status in bariatric surgery patients receiving postoperative supplementation per guidelines: Insights from a systematic review and meta-analysis of longitudinal studies". Obes Rev. 22 (7): e13249. doi:10.1111/obr.13249. PMID 33938111.
- ^ a b c Podszun M, Frank J (December 2014). "Vitamin E-drug interactions: molecular basis and clinical relevance". Nutrition Research Reviews. 27 (2): 215–31. doi:10.1017/S0954422414000146. PMID 25225959. S2CID 38571160.
- ^ "Overview on dietary reference values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). 2017.
- ^ Tolerable upper intake levels For vitamins and minerals (PDF), European Food Safety Authority, 2006
- ^ "Eight key recommendations from Dietary Guidelines for Chinese Residents (2022)". Chinese Center for Disease Control and Prevention. June 2022. Retrieved 23 September 2024.
- ^ "Vitamin E". United Kingdom National Health Services. 23 October 2017. Retrieved 7 January 2022.
- ^ Tanaka K, Terao J, Shidoji Y, Tamai H, Imai E, Okano T (2012). "Dietary reference intakes for Japanese 2010: fat-soluble vitamins". Journal of Nutritional Science and Vitaminology. 59 (Supplement): S57–66. doi:10.3177/jnsv.59.S57.
- ^ "Nutrient requirements and recommended dietary allowances for Indians: a report of the expert group of the Indian Council of Medical Research. pp.283-95 (2009)" (PDF). Archived from the original (PDF) on 15 June 2016. Retrieved 26 February 2018.
- ^ "Federal Register May 27, 2016 food labeling: revision of the nutrition and supplement facts labels. FR page 33982" (PDF).
- ^ "Regulation (EU) No 1169/2011 of the European Parliament and of the Council". Official Journal of the European Union. 22 (11): 18–63. 2011.
- ^ "Unit conversions". National Institutes of Health. Retrieved 21 November 2018.
- ^ "Composition of foods raw, processed, prepared USDA national nutrient database for standard reference, Release 20" (PDF). USDA. February 2008. Archived from the original (PDF) on 19 February 2012.
- ^ "Scientific opinion on dietary reference values for vitamin E as α-tocopherol". EFSA Journal. 13 (7). July 2015. doi:10.2903/j.efsa.2015.4149. S2CID 79232649.
only 2R-α-tocopherol stereoisomers were found to meet human requirements for the vitamin... Currently, only RRR-α-tocopherol is considered to be the physiologically active vitamer.
- ^ Reboul E, Richelle M, Perrot E, Desmoulins-Malezet C, Pirisi V, Borel P (November 2006). "Bioaccessibility of carotenoids and vitamin E from their main dietary sources". Journal of Agricultural and Food Chemistry. 54 (23): 8749–55. doi:10.1021/jf061818s. PMID 17090117.
- ^ a b c d e f g "USDA Food Composition Databases". United States Department of Agriculture, Agricultural Research Service. Release 28. 2015. Archived from the original on 3 March 2018. Retrieved 18 August 2018.
- ^ Tan B, Watson RR, Preedy VR, eds. (2013). Tocotrienols: Vitamin E Beyond Tocopherols (2nd ed.). Boca Raton: CRC Press. ISBN 9781439884416.
- ^ Babura SR, Abdullah SN, Khaza Ai H (2017). "Advances in Genetic Improvement for Tocotrienol Production: A Review". J Nutr Sci Vitaminol (Tokyo). 63 (4): 215–221. doi:10.3177/jnsv.63.215. PMID 28978868.
- ^ "Guidelines on food fortification with micronutrients". World Health Organization. 2006. Retrieved 12 December 2022.
- ^ "Food Fortification Initiative - Why fortify?". Food Fortification Initiative, Enhancing Grains for Better Lives. Retrieved 12 December 2022.
- ^ a b "Scientific opinion on the re-evaluation of tocopherol-rich extract (E 306), α-tocopherol (E 307), γ-tocopherol (E 308) and δ-tocopherol (E 309) as food additives". EFSA Journal. 13 (9). September 2015. doi:10.2903/j.efsa.2015.4247.
- ^ "Frequently asked questions | Why food additives". Food Additives and Ingredients Association UK & Ireland- Making life taste better. Archived from the original on 1 June 2019. Retrieved 27 October 2010.
- ^ Lee GY, Han SN (November 2018). "The Role of Vitamin E in Immunity". Nutrients. 10 (11): 1614. doi:10.3390/nu10111614. PMC 6266234. PMID 30388871.
- ^ a b c d Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, Coker TR, et al. (June 2022). "Vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer: US preventive services task force recommendation statement". JAMA. 327 (23): 2326–33. doi:10.1001/jama.2022.8970. PMID 35727271. S2CID 249886842.
- ^ a b Wang W, Li J, Zhang H, Wang X, Zhang X (January 2021). "Effects of vitamin E supplementation on the risk and progression of AD: a systematic review and meta-analysis". Nutr Neurosci. 24 (1): 13–22. doi:10.1080/1028415X.2019.1585506. PMID 30900960.
- ^ Evans JR, Lawrenson JG (July 2017). "Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration". The Cochrane Database of Systematic Reviews. 2017 (7): CD000253. doi:10.1002/14651858.CD000253.pub4. PMC 6483250. PMID 28756617.
- ^ Dong Y, Chen X, Liu Y, Shu Y, Chen T, Xu L, et al. (February 2018). "Do low-serum vitamin E levels increase the risk of Alzheimer disease in older people? Evidence from a meta-analysis of case-control studies". International Journal of Geriatric Psychiatry. 33 (2): e257–e63. doi:10.1002/gps.4780. PMID 28833475. S2CID 44859128.
- ^ Ashley S, Bradburn S, Murgatroyd C (October 2021). "A meta-analysis of peripheral tocopherol levels in age-related cognitive decline and Alzheimer's disease". Nutr Neurosci. 24 (10): 795–809. doi:10.1080/1028415X.2019.1681066. PMID 31661399.
- ^ Shen C, Huang Y, Yi S, Fang Z, Li L (November 2015). "Association of vitamin E intake with reduced risk of kidney cancer: a meta-analysis of observational Studies". Medical Science Monitor. 21: 3420–6. doi:10.12659/MSM.896018. PMC 4644018. PMID 26547129.
- ^ Wang YY, Wang XL, Yu ZJ (2014). "Vitamin C and E intake and risk of bladder cancer: a meta-analysis of observational studies". International Journal of Clinical and Experimental Medicine. 7 (11): 4154–64. PMC 4276184. PMID 25550926.
- ^ Lotan Y, Goodman PJ, Youssef RF, Svatek RS, Shariat SF, Tangen CM, et al. (June 2012). "Evaluation of vitamin E and selenium supplementation for the prevention of bladder cancer in SWOG coordinated SELECT". The Journal of Urology. 187 (6): 2005–10. doi:10.1016/j.juro.2012.01.117. PMC 4294531. PMID 22498220.
- ^ Zhu YJ, Bo YC, Liu XX, Qiu CG (March 2017). "Association of dietary vitamin E intake with risk of lung cancer: a dose-response meta-analysis". Asia Pacific Journal of Clinical Nutrition. 26 (2): 271–7. doi:10.6133/apjcn.032016.04. PMID 28244705.
- ^ Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group (April 1994). "The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers". The New England Journal of Medicine. 330 (15): 1029–35. doi:10.1056/NEJM199404143301501. PMID 8127329.
- ^ a b Slatore CG, Littman AJ, Au DH, Satia JA, White E (March 2008). "Long-term use of supplemental multivitamins, vitamin C, vitamin E, and folate does not reduce the risk of lung cancer". American Journal of Respiratory and Critical Care Medicine. 177 (5): 524–30. doi:10.1164/rccm.200709-1398OC. PMC 2258445. PMID 17989343.
- ^ Cui R, Liu ZQ, Xu Q (2014). "Blood α-tocopherol, γ-tocopherol levels and risk of prostate cancer: a meta-analysis of prospective studies". PLOS ONE. 9 (3): e93044. Bibcode:2014PLoSO...993044C. doi:10.1371/journal.pone.0093044. PMC 3965522. PMID 24667740.
- ^ Kim Y, Wei J, Citronberg J, Hartman T, Fedirko V, Goodman M (September 2015). "Relation of vitamin E and selenium exposure to prostate cancer risk by smoking Status: A Review and Meta-Analysis". Anticancer Research. 35 (9): 4983–96. PMID 26254398.
- ^ Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, et al. (March 1998). "Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial". Journal of the National Cancer Institute. 90 (6): 440–6. doi:10.1093/jnci/90.6.440. PMID 9521168.
- ^ Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. (October 2011). "Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT)". JAMA. 306 (14): 1549–56. doi:10.1001/jama.2011.1437. PMC 4169010. PMID 21990298.
- ^ Arain MA, Abdul Qadeer A (April 2010). "Systematic review on "vitamin E and prevention of colorectal cancer"". Pakistan Journal of Pharmaceutical Sciences. 23 (2): 125–30. PMID 20363687.
- ^ Lance P, Alberts DS, Thompson PA, Fales L, Wang F, San Jose J, et al. (January 2017). "Colorectal adenomas in participants of the SELECT randomized trial of selenium and vitamin E for prostate cancer prevention". Cancer Prevention Research. 10 (1): 45–54. doi:10.1158/1940-6207.CAPR-16-0104. PMC 5510661. PMID 27777235.
- ^ Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. (July 2005). "Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial". JAMA. 294 (1): 56–65. doi:10.1001/jama.294.1.56. PMID 15998891.
- ^ Jensen SK, Lauridsen C (2007). "Α-Tocopherol Stereoisomers". Alpha-tocopherol stereoisomers. Vitamins & Hormones. Vol. 76. pp. 281–308. doi:10.1016/S0083-6729(07)76010-7. ISBN 978-0-12-373592-8. PMID 17628178.
- ^ "Qualified health claims". Overview from the US Food & Drug Administration. Retrieved 24 August 2018.
- ^ "Alliance for Natural Health v. Sebelius, Case No. 09-1546 (D.D.C.)". US Food & Drug Administration. 2012. Archived from the original on 14 November 2017. Retrieved 24 August 2018.
- ^ a b Zhang Y, Jiang W, Xie Z, Wu W, Zhang D (October 2015). "Vitamin E and risk of age-related cataract: a meta-analysis". Public Health Nutrition. 18 (15): 2804–14. doi:10.1017/S1368980014003115. PMC 10271701. PMID 25591715. S2CID 3168065.
- ^ Mathew MC, Ervin AM, Tao J, Davis RM (June 2012). "Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract". The Cochrane Database of Systematic Reviews. 2012 (6): CD004567. doi:10.1002/14651858.CD004567.pub2. PMC 4410744. PMID 22696344.
- ^ a b c Kirmizis D, Chatzidimitriou D (2009). "Antiatherogenic effects of vitamin E: the search for the Holy Grail". Vascular Health and Risk Management. 5: 767–74. doi:10.2147/vhrm.s5532. PMC 2747395. PMID 19774218.
- ^ Gaziano JM (December 2004). "Vitamin E and cardiovascular disease: observational studies". Annals of the New York Academy of Sciences. 1031 (1): 280–91. Bibcode:2004NYASA1031..280G. doi:10.1196/annals.1331.028. PMID 15753154. S2CID 26369772.
- ^ Loffredo L, Perri L, Di Castelnuovo A, Iacoviello L, De Gaetano G, Violi F (April 2015). "Supplementation with vitamin E alone is associated with reduced myocardial infarction: a meta-analysis". Nutrition, Metabolism, and Cardiovascular Diseases. 25 (4): 354–63. doi:10.1016/j.numecd.2015.01.008. PMID 25779938.
- ^ Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. (November 2008). "Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial". JAMA. 300 (18): 2123–33. doi:10.1001/jama.2008.600. PMC 2586922. PMID 18997197.
- ^ Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. (March 2005). "Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial". JAMA. 293 (11): 1338–47. doi:10.1001/jama.293.11.1338. PMID 15769967.
- ^ Bin Q, Hu X, Cao Y, Gao F (April 2011). "The role of vitamin E (tocopherol) supplementation in the prevention of stroke. A meta-analysis of 13 randomized controlled trials". Thrombosis and Hemostasis. 105 (4): 579–85. doi:10.1160/TH10-11-0729. PMID 21264448. S2CID 23237227.
- ^ a b Maggio E, Bocchini VP, Carnevale R, Pignatelli P, Violi F, Loffredo L (August 2024). "Vitamin E supplementation (alone or with other antioxidants) and stroke: a meta-analysis". Nutr Rev. 82 (8): 1069–78. doi:10.1093/nutrit/nuad114. PMID 37698992.
- ^ "Letter regarding dietary supplement health claim for vitamin E and heart disease (Docket No 99P-4375)". U.S. Food and Drug Administration. Archived from the original on 15 November 2017. Retrieved 24 August 2018.
- ^ "Scientific Opinion on the substantiation of health claims related to vitamin E and protection of DNA, proteins and lipids from oxidative damage (ID 160, 162, 1947), maintenance of the normal function of the immune system (ID 161, 163), maintenance of normal bone (ID 164), maintenance of normal teeth (ID 164), maintenance of normal hair (ID 164), maintenance of normal skin (ID 164), maintenance of normal nails (ID 164), maintenance of normal cardiac function (ID 166), maintenance of normal vision by protection of the lens of the eye (ID 167), contribution to normal cognitive function (ID 182, 183), regeneration of the reduced form of vitamin C (ID 203), maintenance of normal blood circulation (ID 216) and maintenance of normal a scalp (ID 2873) pursuant to Article 13(1) of Regulation (EC) No 1924/2006". EFSA Journal. 8 (10): 1816. 2010. doi:10.2903/j.efsa.2010.1816.
- ^ Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, et al. (2015). "Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials". Nutrition. 31 (7–8): 923–30. doi:10.1016/j.nut.2014.11.018. PMID 26059365.
- ^ Vadarlis A, Antza C, Bakaloudi DR, Doundoulakis I, Kalopitas G, Samara M, et al. (February 2021). "Systematic review with meta-analysis: The effect of vitamin E supplementation in adult patients with non-alcoholic fatty liver disease". J Gastroenterol Hepatol. 36 (2): 311–19. doi:10.1111/jgh.15221. PMID 32810309. S2CID 221181369.
- ^ Wang MY, Prabahar K, Găman MA, Zhang JL (2023). "Vitamin E supplementation in the treatment on nonalcoholic fatty liver disease (NAFLD): Evidence from an umbrella review of meta-analysis on randomized controlled trials". J Dig Dis. 24 (6–7): 380–89. doi:10.1111/1751-2980.13210. PMID 37503812.
- ^ Amanullah I, Khan YH, Anwar I, Gulzar A, Mallhi TH, Raja AA (November 2019). "Effect of vitamin E in non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials". Postgrad Med J. 95 (1129): 601–11. doi:10.1136/postgradmedj-2018-136364. PMID 31434683. S2CID 201275520.
- ^ Lin M, Zeng H, Deng G, Lei J, Li J (May 2021). "Vitamin E in pediatric non-alcoholic fatty liver disease: a meta-analysis". Clin Res Hepatol Gastroenterol. 45 (3): 101530. doi:10.1016/j.clinre.2020.08.008. PMID 33272889. S2CID 227282863.
- ^ de Lima KS, Schuch F, Righi NC, Neto LJ, Nunes GS, Puntel G, et al. (June 2024). "Vitamin E Does not Favor Recovery After Exercises: Systematic Review and Meta-analysis". Int J Sports Med. 45 (7): 485–95. doi:10.1055/a-2221-5688. PMID 38346687.
- ^ Etminan M, Gill SS, Samii A (June 2005). "Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson's disease: a meta-analysis". The Lancet. Neurology. 4 (6): 362–5. doi:10.1016/S1474-4422(05)70097-1. PMID 15907740. S2CID 25691968.
- ^ Chang MC, Kwak SG, Kwak S (June 2021). "Effect of dietary vitamins C and E on the risk of Parkinson's disease: A meta-analysis". Clin Nutr. 40 (6): 3922–30. doi:10.1016/j.clnu.2021.05.011. PMID 34139465. S2CID 235470579.
- ^ "Vitamin E and C supplementation during pregnancy". www.who.int. August 2023. Retrieved 21 October 2024.
- ^ Rumbold A, Ota E, Hori H, Miyazaki C, Crowther CA (September 2015). "Vitamin E supplementation in pregnancy". The Cochrane Database of Systematic Reviews. 2016 (9): CD004069. doi:10.1002/14651858.CD004069.pub3. PMC 8406700. PMID 26343254.
- ^ a b Panin G, Strumia R, Ursini F (December 2004). "Topical alpha-tocopherol acetate in the bulk phase: eight years of experience in skin treatment". Annals of the New York Academy of Sciences. 1031 (1): 443–7. Bibcode:2004NYASA1031..443P. doi:10.1196/annals.1331.069. PMID 15753192. S2CID 45771699.
- ^ a b Sidgwick GP, McGeorge D, Bayat A (August 2015). "A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring". Archives of Dermatological Research. 307 (6): 461–77. doi:10.1007/s00403-015-1572-0. PMC 4506744. PMID 26044054.
- ^ a b Tanaydin V, Conings J, Malyar M, van der Hulst R, van der Lei B (September 2016). "The role of topical vitamin E in scar management: a systematic review". Aesthetic Surgery Journal. 36 (8): 959–65. doi:10.1093/asj/sjw046. PMID 26977069.
- ^ Kosari P, Alikhan A, Sockolov M, Feldman SR (2010). "Vitamin E and allergic contact dermatitis". Dermatitis. 21 (3): 148–53. doi:10.2310/6620.2010.09083. PMID 20487657. S2CID 38212099.
- ^ Outbreak of lung injury associated with e-cigarette use, or 'vaping' (Report). Centers for Disease Control and Prevention. 11 February 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Sun L (6 September 2019). "Contaminant found in marijuana vaping products linked to deadly lung illnesses, tests show". The Washington Post. Retrieved 9 September 2019.
- ^ Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, et al. (February 2020). "Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI". N Engl J Med. 382 (8): 697–705. doi:10.1056/NEJMoa1916433. PMC 7032996. PMID 31860793.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ "Transcript of CDC telebriefing: update on lung injury associated with e-cigarette use, or vaping". Centers for Disease Control and Prevention. 8 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Boudi FB, Patel S, Boudi A, Chan C (December 2019). "Vitamin E acetate as a plausible cause of acute vaping-related illness". Cureus. 11 (12): e6350. doi:10.7759/cureus.6350. PMC 6952050. PMID 31938636.
- ^ Wu D, O'Shea DF (March 2020). "Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate". Proceedings of the National Academy of Sciences of the United States of America. 117 (12): 6349–55. Bibcode:2020PNAS..117.6349W. doi:10.1073/pnas.1920925117. PMC 7104367. PMID 32156732.
- ^ Evans HM, Bishop KS (December 1922). "On the existence of a hitherto unrecognized dietary factor essential for reproduction". Science. 56 (1458): 650–1. Bibcode:1922Sci....56..650E. doi:10.1126/science.56.1458.650. JSTOR 1647181. PMID 17838496.
- ^ Oakes EH (2007), "Emerson, Gladys Anderson", Encyclopedia of world scientists, Infobase, pp. 211–2, ISBN 978-1-4381-1882-6
- ^ Evans HM, Emerson OH, Emerson GA. (1936). "The isolation from wheat germ oil of an alcohol, a-tocopherol, having the properties of vitamin E". Journal of Biological Chemistry. 113 (1): 319–2. doi:10.1016/S0021-9258(18)74918-1.
- ^ Karrer P, Fritzsche H, Ringier BH, Salomon H (1938). "Synthesis of α-tocopherol (vitamin E)". Nature. 141 (3580): 1057. Bibcode:1938Natur.141.1057K. doi:10.1038/1411057d0. S2CID 4118327.
- ^ "Vitamin in search of a disease". JAMA: The Journal of the American Medical Association. 201 (3): 195–6. 1967. doi:10.1001/jama.1967.03130030065018.
- ^ Vogelsang A, Shute EV (June 1946). "Effect of vitamin E in coronary heart disease". Nature. 157 (3997): 772. Bibcode:1946Natur.157..772V. doi:10.1038/157772b0. PMID 21064771. S2CID 4099854.
- ^ Skelton F, Shute E, Skinner HG, Waud RA (June 1946). "Antipurpuric action of α-tocopherol (vitamin E)". Science. 103 (2687): 762. Bibcode:1946Sci...103R.762S. doi:10.1126/science.103.2687.762-b. PMID 17836459. S2CID 35677118.
- ^ Shute EV, Vogelsang AB (January 1948). "The influence of vitamin E on vascular disease". Surgery, Gynecology & Obstetrics. 86 (1): 1–8. PMID 18920873.
- ^ Shute WE, Shute EV. Alpha-tocopherol (vitamin E) in cardiovascular disease Toronto, Ontario, Canada: Ryerson Press, 1954
- ^ Eidelman RS, Hollar D, Hebert PR, Lamas GA, Hennekens CH (July 2004). "Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease". Archives of Internal Medicine. 164 (14): 1552–6. doi:10.1001/archinte.164.14.1552. PMID 15277288.
- ^ Bell EF (July 1987). "History of vitamin E in infant nutrition". The American Journal of Clinical Nutrition. 46 (1 Suppl): 183–6. doi:10.1093/ajcn/46.1.183. PMID 3300257.
- ^ Brion LP, Bell EF, Raghuveer TS (2003). "Vitamin E supplementation for prevention of morbidity and mortality in preterm infants". The Cochrane Database of Systematic Reviews. 2010 (4): CD003665. doi:10.1002/14651858.CD003665. PMC 8725195. PMID 14583988.
