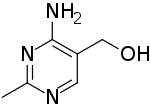
4-Amino-5-hydroxymethyl-2-methylpyrimidine
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
(4-Amino-2-methylpyrimidin-5-yl)methanol | |
| Other names
HMP
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII |
|
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Within the field of biochemistry, 4-amino-5-hydroxymethyl-2-methylpyrimidine (HMP) also known as toxopyrimidine together with its mono phosphate (HMP-P) and pyrophosphate (HMP-PP) esters are biogenetic precursors to the important biochemical cofactor thiamine pyrophosphate (TPP), a derivative of thiamine (vitamin B1).
HMP, HMP-P and HMP-PP are found along with thiamine forms in a wide variety of living organisms. Thiamine in various salt, formulation and biological matrix forms are used to supplement human and animal diets because these organisms lack the capability to produce it. Methodologies are being sought for biotechnology-based production of thiamine forms and for increasing thiamine content in food sources.
TPP biogenesis
[edit]In microorganisms and plants TPP results from coupling of pyrimidine fragment HMP-PP with thiazole fragment HET-P to give thiamine monophosphate, followed by conversion to the pyrophosphate.[1][2]
Biogenesis of HMP-P and HET-P vary with types of organism.
HMP-P biogenesis
[edit]In bacteria, HMP-P arises by conversion of the purine biosynthetic precursor 5-aminoimidazole ribotide (AIR) through the action of enzymes such as phosphomethylpyrimidine synthase, a member of the radical SAM superfamily.[3][4] Studies using isotopically labelled AIR have shown which atoms carry into the product.[5][6] Mechanisms by which this occurs are not yet known with certainty.
In yeasts, HMP-P is derived from metabolites of histidine and pyridoxine.[7][8] Some of these transformations appear to be catalyzed by radical SAM enzymes. Isotopically labelled precursors have been used to investigate this biogenesis.[5][9][10] Mechanisms of the transformations are unknown.
In Salmonella, HMP-P can be derived independently of purine biogenesis when AICAR is available.[11][12]
In algae, thiamine forms and precursors are scavenged by uptake from water of exogenous products from other organisms. In higher plants, thiamine biogenesis resembles that of bacteria.[2][13] In some circumstances, thiamine forms and precursors may be obtained through symbiotic relationships with microorganisms in the soil.
Genes relevant for transformations in the biogenesis of HMP-P, HET-P, and TPP have been identified in various organisms and some of the proteins resulting from their expression have been characterized.[14][15] Biosynthesis of TPP is feedback inhibited through actions of a riboswitch.[16]
Research is ongoing towards understanding biochemistry involved and towards facilitating technologies of socioeconomic value for supply of thiamine in various forms.
Related technologies
[edit]Commercially available salts thiamine chloride and thiamine nitrate are produced at scales of thousands of tons annually by chemistry-based manufacturing processes in Europe and Asia.[17][18] These salts are supplied for formulations for supplementation of human diet and as feed additives for cattle, swine, poultry and fish.
Research for potential biotechnology-based production of thiamine[19][20][21] has resulted in patent applications claiming fermentation using recombinant microorganisms modified to deregulate feedback inhibition and allow release of thiamine forms to the media as demonstrated at small scale.[22][23]
Thiamine forms and their bio-precursors are produced at very large scale in biological matrices such as yeast, grains, plants and meats widely consumed as food and feed. Research into genetic modification of plants.[24] has led to higher levels of thiamine in foodstuffs, such as rice.[25] Use of thiamine forms and their bio-precursors by various means such as seed coating or soil and foliar fertilization to improve plant growth and properties are being investigated.[26]
References
[edit]- ^ Jurgenson CT, Begley TP, Ealick SE (2009). "The structural and biochemical foundations of thiamin biosynthesis". Annual Review of Biochemistry. 78 (1): 569–603. doi:10.1146/annurev.biochem.78.072407.102340. PMC 6078420. PMID 19348578.
- ^ a b Roje S (July 2007). "Vitamin B biosynthesis in plants". Phytochemistry. 68 (14): 1904–21. Bibcode:2007PChem..68.1904R. doi:10.1016/j.phytochem.2007.03.038. PMID 17512961.
- ^ Broderick JB, Duffus BR, Duschene KS, Shepard EM (April 2014). "Radical S-adenosylmethionine enzymes". Chemical Reviews. 114 (8): 4229–317. doi:10.1021/cr4004709. PMC 4002137. PMID 24476342.
- ^ Chatterjee A, Li Y, Zhang Y, Grove TL, Lee M, Krebs C, Booker SJ, Begley TP, Ealick SE (December 2008). "Reconstitution of ThiC in thiamine pyrimidine biosynthesis expands the radical SAM superfamily". Nature Chemical Biology. 4 (12): 758–65. doi:10.1038/nchembio.121. PMC 2587053. PMID 18953358.
- ^ a b Spenser ID, White RL (May 1997). "Biosynthesis of vitamin B1 (thiamin): an instance of biochemical diversity". Angewandte Chemie International Edition in English. 36 (10): 1032–46. doi:10.1002/anie.199710321.
- ^ Begley TP, Chatterjee A, Hanes JW, Hazra A, Ealick SE (April 2008). "Cofactor biosynthesis--still yielding fascinating new biological chemistry". Current Opinion in Chemical Biology. 12 (2): 118–25. doi:10.1016/j.cbpa.2008.02.006. PMC 2677635. PMID 18314013.
- ^ Zeidler J, Sayer BG, Spenser ID (October 2003). "Biosynthesis of vitamin B1 in yeast. Derivation of the pyrimidine unit from pyridoxine and histidine. Intermediacy of urocanic acid". Journal of the American Chemical Society. 125 (43): 13094–105. doi:10.1021/ja030261j. PMID 14570482.
- ^ Lai RY, Huang S, Fenwick MK, Hazra A, Zhang Y, Rajashankar K, et al. (June 2012). "Thiamin pyrimidine biosynthesis in Candida albicans : a remarkable reaction between histidine and pyridoxal phosphate". Journal of the American Chemical Society. 134 (22): 9157–9. doi:10.1021/ja302474a. PMC 3415583. PMID 22568620.
- ^ Lawhorn BG, Mehl RA, Begley TP (September 2004). "Biosynthesis of the thiamin pyrimidine: the reconstitution of a remarkable rearrangement reaction". Organic & Biomolecular Chemistry. 2 (17): 2538–46. doi:10.1039/B405429F. PMID 15326535.
- ^ Himmeldirk K, Sayer BG, Spenser ID (April 1998). "Comparative biogenetic anatomy of vitamin B1: a 13C NMR investigation of the biosynthesis of thiamin in Escherichia coli and in Saccharomyces cerevisiae". Journal of the American Chemical Society. 120 (15): 3581–9. doi:10.1021/ja973835r.
- ^ Bazurto JV, Downs DM (February 2011). "Plasticity in the purine-thiamine metabolic network of Salmonella". Genetics. 187 (2): 623–31. doi:10.1534/genetics.110.124362. PMC 3030501. PMID 21135073.
- ^ Bazurto JV, Heitman NJ, Downs DM (September 2015). Metcalf WW (ed.). "Aminoimidazole Carboxamide Ribotide Exerts Opposing Effects on Thiamine Synthesis in Salmonella enterica". Journal of Bacteriology. 197 (17): 2821–30. doi:10.1128/JB.00282-15. PMC 4524041. PMID 26100042.
- ^ Goyer A (October 2010). "Thiamine in plants: aspects of its metabolism and functions". Phytochemistry. 71 (14–15): 1615–24. Bibcode:2010PChem..71.1615G. doi:10.1016/j.phytochem.2010.06.022. PMID 20655074.
- ^ Jurgenson CT, Ealick SE, Begley TP (August 2009). "Biosynthesis of Thiamin Pyrophosphate". EcoSal Plus. 3 (2). doi:10.1128/ecosalplus.3.6.3.7. PMC 6039189. PMID 26443755.
- ^ Settembre E, Begley TP, Ealick SE (December 2003). "Structural biology of enzymes of the thiamin biosynthesis pathway". Current Opinion in Structural Biology. 13 (6): 739–47. doi:10.1016/j.sbi.2003.10.006. PMID 14675553.
- ^ Bocobza SE, Aharoni A (October 2008). "Switching the light on plant riboswitches". Trends in Plant Science. 13 (10): 526–33. Bibcode:2008TPS....13..526B. doi:10.1016/j.tplants.2008.07.004. PMID 18778966.
- ^ Eggersdorfer M, Laudert D, Létinois U, McClymont T, Medlock J, Netscher T, Bonrath W (December 2012). "One hundred years of vitamins-a success story of the natural sciences". Angewandte Chemie. 51 (52): 12960–90. doi:10.1002/anie.201205886. PMID 23208776.
- ^ Burdick D (2000). "Thiamine (B1)". Kirk-Othmer Encyclopedia of Chemical Technology. American Cancer Society. doi:10.1002/0471238961.2008090102211804.a01. ISBN 9780471238966.
- ^ Revuelta JL, Buey RM, Ledesma-Amaro R, Vandamme EJ (September 2016). "Microbial biotechnology for the synthesis of (pro)vitamins, biopigments and antioxidants: challenges and opportunities". Microbial Biotechnology. 9 (5): 564–7. doi:10.1111/1751-7915.12379. PMC 4993173. PMID 27373767.
- ^ Hanson AD, Amthor JS, Sun J, Niehaus TD, Gregory JF, Bruner SD, Ding Y (August 2018). "Redesigning thiamin synthesis: Prospects and potential payoffs". Plant Science. 273: 92–99. Bibcode:2018PlnSc.273...92H. doi:10.1016/j.plantsci.2018.01.019. PMID 29907313. S2CID 49217720.
- ^ Acevedo-Rocha CG, Gronenberg LS, Mack M, Commichau FM, Genee HJ (August 2018). "Microbial cell factories for the sustainable manufacturing of B vitamins". Current Opinion in Biotechnology. 56: 18–29. doi:10.1016/j.copbio.2018.07.006. PMID 30138794.
- ^ WO application 2017103221, Gronenberg L, Ferla M, Genee M, "A Genetically Modified Bacterial Cell Factory for Thiamine Production", published 22 June 2017, assigned to Biosyntia APS
- ^ US application 2009233296, Goese M, Perkins J, Schyns G, "Thiamin production by fermentation", published 17 September 2009, assigned to DSM IP Assets B.V.
- ^ Goyer A (April 2017). "Thiamin biofortification of crops". Current Opinion in Biotechnology. 44: 1–7. doi:10.1016/j.copbio.2016.09.005. PMID 27750185.
- ^ Dong W, Thomas N, Ronald PC, Goyer A (2016). "Overexpression of Thiamin Biosynthesis Genes in Rice Increases Leaf and Unpolished Grain Thiamin Content But Not Resistance to Xanthomonas oryzae pv. oryzae". Frontiers in Plant Science. 7: 616. doi:10.3389/fpls.2016.00616. PMC 4861732. PMID 27242822.
- ^ Ahn IP, Kim S, Lee YH (July 2005). "Vitamin B1 functions as an activator of plant disease resistance". Plant Physiology. 138 (3): 1505–15. doi:10.1104/pp.104.058693. PMC 1176421. PMID 15980201.
