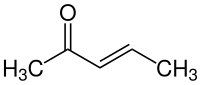
3-Penten-2-one
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pent-3-en-2-one | |
| Other names
Ethylidene acetone
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3-01-00-02985 | |
| ChemSpider | |
| ECHA InfoCard | 100.009.899 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H8O | |
| Molar mass | 84.118 g·mol−1 |
| Appearance | colourless liquid[1] |
| Density | 0.861 g·cm−3[1] 0,862 g·cm−3 (E)[2] |
| Boiling point | 122 °C (252 °F; 395 K) |
| soluble in water, acetone and ether (E)[3] | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable and toxic |
| GHS labelling: | |
 
| |
| Danger | |
| H225, H312, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P312, P321, P322, P332+P313, P337+P313, P362, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Safety data sheet (SDS) | Safety Data Sheet |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
3-Penten-2-one is an organic compound with the formula CH3C(O)CH=CHCH3. It exists as (E) and (Z) stereoisomers. The compound is classified as an α,β-unsaturated ketone. It is a colorless volatile liquid with fruity to pungent odor.[4][5]
Preparation, occurrence, uses
[edit]The (E) isomer is classically obtained from the 3-chloropentanone by dehydrohalogenation.[6] It can also be obtained by dehydration of 4-hydroxy-pentan-2-one using oxalic acid as a catalyst.[5]
3-Penten-2-one occurs naturally in the berries of two species of Aronia melanocarpa.[7] It has also been found in other plants and foods such as tomatoes, cocoa, tea, and potato chips.[5]
3-Penten-2-one can be used for the synthesis of other compounds such as the alkaloids senepodine G and cermizine C, for example.[7] It is also a useful flavoring agent.[5]
References
[edit]- ^ a b Record of 3-Penten-2-on in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 25 July 2017.
- ^ Carl L. Yaws (2015), The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals Physical Properties for More Than 54,000 Organic and Inorganic Chemical Compounds, Coverage for C1 to C100 Organics and Ac to Zr Inorganics, Gulf Professional Publishing, p. 72, ISBN 978-0-12-801146-1
- ^ William M. Haynes (2016), CRC Handbook of Chemistry and Physics (97th ed.), CRC Press, p. 440, ISBN 978-1-4987-5429-3
- ^ "L13031 3-Penten-2-one, tech. 85%". Alfa Aesar.
- ^ a b c d George A. Burdock (1997), Encyclopedia of Food and Color Additives, CRC Press, p. 2997, ISBN 978-0-8493-9414-0
- ^ H. C. Odom & A. R. Pinder (1971). "trans-3-Penten-2-one". Org. Synth. 51: 115. doi:10.15227/orgsyn.051.0115.
- ^ a b Sigma-Aldrich Co., product no. {{{id}}}.
