2.2.2-Propellane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Tricyclo[2.2.2.01,4]octane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H12 | |||
| Molar mass | 108.184 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
[2.2.2]Propellane, formally tricyclo[2.2.2.01,4]octane is an organic compound, a member of the propellane family. It is a hydrocarbon with formula C8H12, or C2(C2H4)3. Its molecule has three rings with four carbon atoms each, sharing one C–C bond.
This compound is unstable (although not as much as [1.1.1]propellane; however it is less persistent than [1.1.1]propellane[1]). The bond angles on the shared carbons are considerably strained: three of them are close to 90°, the other three to 120°. The strain energy is estimated to be 93 kcal/mol (390 kJ/mol).
Synthesis
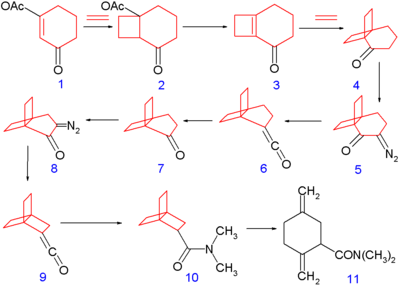
[edit][2.2.2]Propellane was first synthesized in 1973 by the group of Philip Eaton (who had earlier obtained cubane),[2] according to the following scheme:
The synthesis begins with photochemical [2+2]cycloaddition of ethene on the cyclohexene derivative 1 to produce the bicyclic compound 2, followed by elimination reaction with potassium t-butoxide of acetic acid to cyclobutene 3, followed by another cycloaddition with ethylene to 4. This compound is converted to the diazo ketone 5 by deprotonation (using acetic acid and sodium methoxide) and reaction with tosyl azide. The ketone then undergoes Wolff rearrangement to ketene 6. Ozonolysis forms the ketone 7, another diazotation yields the diazo ketone 8, which undergoes Wolff rearrangement again to the ketene 9. Reaction with dimethylamine affords the [2.2.2]propellane backbone with a dimethylamide substituent 10.
The final product 10 was found to spontaneously isomerize in solution to the monocyclic amide 11, with a half-life of 28 minutes at room temperature.
Derivatives
[edit]A highly fluorinated [2.2.0]propellane was also synthesized by the group of David Lemal.[3]
See also
[edit]References
[edit]- ^ "Houben-Weyl Methods of Organic Chemistry Vol. E 17e, 4th Edition Supplement (E-Book PDF) - Thieme.de - Thieme Webshop - Armin de Meijere, Holger Butenschön, Hak-Fun Chow, Lutz Fitjer, Günter Haufe". Thieme Webshop (in German). Archived from the original on October 22, 2017. Retrieved 2017-10-21.
- ^ Eaton, Philip E.; Temme, George H. (1973). "[2.2.2]Propellane system". J. Am. Chem. Soc. 95 (22): 7508–7510. doi:10.1021/ja00803a052.
- ^ Zhang, Y.; Smith, J.R.; Lemal, D.M. (1996). "Octafluorobicyclo[2.2.0]hex-1(4)-ene: A Greatly Strained Alkene with Novel Reactivity". J. Am. Chem. Soc. 118 (39): 9454. doi:10.1021/ja961656o.