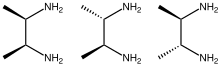
2,3-Butanediamine
 | |
| Names | |
|---|---|
| Other names
1,2-Dimethylethylenediamine
2,3-Diaminobutane Butane-2,3-diamine | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H12N2 | |
| Molar mass | 88.154 g·mol−1 |
| Appearance | colorless oil |
| Boiling point | 44-45 °C (25 mmHg, rac) 46-48 °C (25 mmHg, meso)[1] 55.3-59.3 °C (60 mmHg, DL-threo) 56.1-60.5 °C (60 mmHg, meso)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2,3-Butanediamine are organic compounds with the formula CH3CH(NH2)CH(NH2)CH3. Three stereoisomers exist, meso and a pair of enantiomers. These diamines form complexes with transition metals.[3]
Synthesis
[edit]2,3-Butanediamines can be prepared by hydrolyzing 2-ethoxy-4,5-dihydro-4,5-dimethylimidazole with barium hydroxide.[4] Alternative, it is produced by reduction of dimethylglyoxime with lithium aluminium hydride.[5] The meso and the d,l diastereomers can be separated by fractional crystallization of the hydrochlorides. The enantiomers have been resolved using tartrate salts.[6]
Reactions
[edit]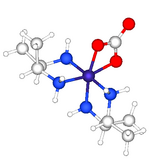
In coordination chemistry, 2,3-butanediamine (abbreviated bn) has illuminates aspects of the stereochemistry. The structure of [Co(meso-2,3-butanediamine)2CO3]+ confirms the presence of the rarely observed axial methyl groups on each of the diamine-cobalt rings.[7]
Related compounds
[edit]- 1,2-Diaminopropane, chiral 1,2-diamine
- 1,2-Diaminocyclohexane, a 1,2-diamine that also exists as three stereoisomers
References
[edit]- ^ Fred Basolo, R. Kent Murmann, and Yun Ti Chen. Dissociation Constants of Substituted Ethylenediamines. J. Am. Chem. Soc. 1953, 75, 6, 1478–1480. doi:10.1021/ja01102a507.
- ^ Robert Ghirardelli and Howard J. Lucas. Stereochemistry of the Opening of the Imine Ring with Ethylamine. J. Am. Chem. Soc. 1957, 79, 3, 734–741. doi:10.1021/ja01560a064.
- ^ Tsuchiya, Ryokichi; Uehara, Akira; Yoshikuni, Tadatsugu (1982). "Solid-phase thermal cis-trans isomerization of bis(diamine)chromium(III) complexes containing d,l-2,3-butanediamine, d,l-1,2-cyclohexanediamine, or d,l-2,4-pentanediamine". Inorganic Chemistry. 21 (2): 590–594. doi:10.1021/ic00132a025.
- ^ Harold Kohn and Sang Hun Jung. New stereoselective method for the preparation of vicinal diamines from olefins and cyanamide. Journal of the American Chemical Society 1983 105 (12), 4106-4108. doi:10.1021/ja00350a068.
- ^ Hilleary, Christopher J.; Them, Theodore F.; Tapscott, Robert E. (1980). "Stereochemical studies on diastereomers of tris(2,3-butanediamine)cobalt(III)". Inorganic Chemistry. 19: 102–107. doi:10.1021/ic50203a022.
- ^ Dickey, F. H.; Fickett, Wildon; Lucas, H. J. (1952). "Stereoisomeric 2,3-Butanediamines, 3-Amino-2-butanols and 2,3-Dimethylethyleneimines; Stereochemistry of the Opening and Closing of the Imine Ring1". Journal of the American Chemical Society. 74 (4): 944–951. Bibcode:1952JAChS..74..944D. doi:10.1021/ja01124a023.
- ^ Duesler, E. N.; Fe Gargallo, M.; Tapscott, R. E. (1982). "Structure of lel lel lel tris[(±)-2,3-butanediamine]-cobalt(III) chloride". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 38 (4): 1300–1303. Bibcode:1982AcCrB..38.1300D. doi:10.1107/S0567740882005585.
