CYP17A1
| Steroid 17-alpha-hydroxylase/17,20 lyase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 1.14.14.19 | ||||||||
| CAS no. | 9029-67-8 | ||||||||
| Alt. names | P450c17, CYP17A1, steroid 17-alpha-hydroxylase/17,20 lyase, CYPXVII, cytochrome P450 17A1, cytochrome p450 XVIIA1, cytochrome P450-C17, cytochrome P450, family 17, subfamily A, polypeptide 1, steroid 17-alpha-monooxygenase, cytochrome P450c17, 4.1.2.30, 17-alpha-hydroxyprogesterone aldolase, cytochrome P450, subfamily XVII (steroid 17-alpha-hydroxylase), steroid 17 alpha-hydroxylase/17,20 lyase, IPR033282 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Cytochrome P450 17A1 (steroid 17α-monooxygenase, 17α-hydroxylase, 17-alpha-hydroxylase, 17,20-lyase, 17,20-desmolase) is an enzyme of the hydroxylase type that in humans is encoded by the CYP17A1 gene on chromosome 10.[5] It is ubiquitously expressed in many tissues and cell types, including the zona reticularis and zona fasciculata (but not zona glomerulosa) of the adrenal cortex as well as gonadal tissues.[6][7] It has both 17α-hydroxylase and 17,20-lyase activities, and is a key enzyme in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens. More specifically, the enzyme acts upon pregnenolone and progesterone to add a hydroxyl (-OH) group at carbon 17 position (C17) of the steroid D ring (the 17α-hydroxylase activity, EC 1.14.14.19), or acts upon 17α-hydroxyprogesterone and 17α-hydroxypregnenolone to split the side-chain off the steroid nucleus (the 17,20-lyase activity, EC 1.14.14.32).[7]
Structure
[edit]Gene
[edit]The CYP17A1 gene resides on chromosome 10 at the band 10q24.3 and contains 8 exons.[5] The cDNA of this gene spans a length of 1527 bp.[8] This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are generally regarded as monooxygenases that catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids, and other lipids, including the remarkable carbon-carbon bond scission catalyzed by this enzyme.
The CYP17A1 gene may also contain variants associated with increased risk of coronary artery disease.[9][non-primary source needed]
Protein
[edit]CYP17A1 is a 57.4 kDa protein that belongs to the cytochrome P450 family.[10][11] The protein encoded by its cDNA is composed of 508 amino acid residues. As an enzyme, CYP17A1 possesses an active site that associates with a heme prosthetic group to catalyze biosynthetic reactions.[8] Based on its known structures while bound to two steroidal inhibitors, abiraterone and galeterone, CYP17A1 possesses the canonical cytochrome P450 fold present in other complex P450 enzymes that participate in steroidogenesis or cholesterol metabolism, though it orients the steroid ligands toward the F and G helices, perpendicular to the heme group, rather than the β1 sheet.[12][13]
Expression
[edit]Expression of CYP17A1 has been found in all of the traditional steroidogenic tissues except the placenta, including the zona reticularis and zona fasciculata of the adrenal cortex, the Leydig cells of the testes, the thecal cells of the ovaries, and, more recently, in luteinized granulosa cells in ovarian follicles.[14] In addition to classical steroidogenic tissue, CYP17A1 has also been detected in the heart, kidney, and adipose tissue.[14] In the fetus, CYP17A1 has been reported in the kidney, thymus, and spleen.[14]
Function
[edit]CYP17A1 is a member of the cytochrome P450 superfamily of enzymes localized in the endoplasmic reticulum. Proteins in this family are monooxygenases that catalyze synthesis of cholesterol, steroids and other lipids and are involved in drug metabolism.[5] CYP17A1 has both 17α-hydroxylase activity (EC 1.14.14.19) and 17,20-lyase activity (EC 1.14.14.32). The 17α-hydroxylase activity of CYP17A1 is required for the generation of glucocorticoids such as cortisol, but both the hydroxylase and 17,20-lyase activities of CYP17A1 are required for the production of androgenic and oestrogenic sex steroids by converting 17α-hydroxypregnenolone to dehydroepiandrosterone (DHEA).[15] Mutations in this gene are associated with isolated steroid-17α-hydroxylase deficiency, 17α-hydroxylase/17,20-lyase deficiency, pseudohermaphroditism, and adrenal hyperplasia.[5]
Furthermore, the 17,20-lyase activity is dependent on cytochrome P450 oxidoreductase (POR) cytochrome b5 (CYB5) and phosphorylation.[16][17][18] Cytochrome b5 acts as a facilitator for 17,20 lyase activity of CYP17A1 and can donate a second electron to some P450s. In humans the production of testosterone via pregnenolone to17-OHPreg and DHEA by the CYP17A1 requires POR.[19][20] Human CYP17A1 protein is phosphorylated on serine and threonine residues by a cAMP-dependent protein kinase. Phosphorylation of the protein increases 17,20-lyase activity, while dephosphorylation virtually eliminates this activity.[18]
Clinical significance
[edit]Mutations in this gene are associated with rare forms of congenital adrenal hyperplasia, specifically 17α-hydroxylase deficiency/17,20-lyase deficiency and isolated 17,20-lyase deficiency.[21]
In humans, the CYP17A1 gene is largely associated with endocrine effects and steroid hormone metabolism.[22][23][24] Furthermore, mutations in the CYP17A1 gene are associated with rare forms of congenital adrenal hyperplasia, in particular 17α-hydroxylase deficiency/17,20-lyase deficiency and isolated 17,20-lyase deficiency. Overall, CYP17A1 is an important target for inhibition in the treatment of prostate cancer because it produces androgen that is required for tumor cell growth.[25][26] The decreased enzyme activity of CYP17A1 is related to infertility due to hypogonadotropic hypogonadism. In females, folliculogenesis is arrested, while in males, testicular atrophy with interstitial cell proliferation and arrested spermatogenesis. Although generally anovulatory, there are some case reports of women with 17α-hydroxylase deficiency who underwent spontaneous menarche with cyclic menses.[27]
Clinical marker
[edit]A multi-locus genetic risk score study based on a combination of 27 loci, including the CYP17A1 gene, identified individuals at increased risk for both incident and recurrent coronary artery disease events, as well as an enhanced clinical benefit from statin therapy. The study was based on a community cohort study (the Malmo Diet and Cancer study) and four additional randomized controlled trials of primary prevention cohorts (JUPITER and ASCOT) and secondary prevention cohorts (CARE and PROVE IT-TIMI 22).[9]
As a drug target
[edit]CYP17A1 inhibitors
[edit]In 2011, the FDA approved the CYP17A1 inhibitor, abiraterone, which contains a steroidal scaffold that is similar to the endogenous CYP17A1 substrates, with prednisone for the treatment of castration-resistant prostate cancer. Abiraterone is structurally similar to the substrates of other cytochrome P450 enzymes involved in steroidogenesis, and interference can pose a liability in terms of side effects. Using nonsteroidal scaffolds is expected to enable the design of compounds that interact more selectively with CYP17A1.[26] Potent inhibitors of the CYP17A1 enzyme provide a last line defense against ectopic androgenesis in advanced prostate cancer.[28]
The drug abiraterone acetate, which is used to treat castration-resistant prostate cancer, blocks the biosynthesis of androgens by inhibiting the CYP17A1 enzyme. Abiraterone acetate binds in the active site of the enzyme[29] and coordinates the heme iron through its pyridine nitrogen, mimicking the substrate.[30]
Since 2014, galeterone has been in phase III clinical trials for castration-resistant prostate cancer.[31]
Ketoconazole is an older CYP17A1 inhibitor that is now little used. However, ketoconazole competitively inhibits CYP17A1, therefore its effectiveness will depend on the concentration of ketoconazole. This is in contrast to the abiraterone acetate, that permanently (rather than competitively) disables CYP17A1, once it binds to it.
Seviteronel (VT-464) is a novel CYP17A1 inhibitor which is aimed to avoid co-administration of glucocorticoid therapy.[32] In the 2010s, it underwent various phases of clinical studies and preclinical models as a drug against prostate cancer or breast cancer.[33][34]
Steroidogenesis
[edit]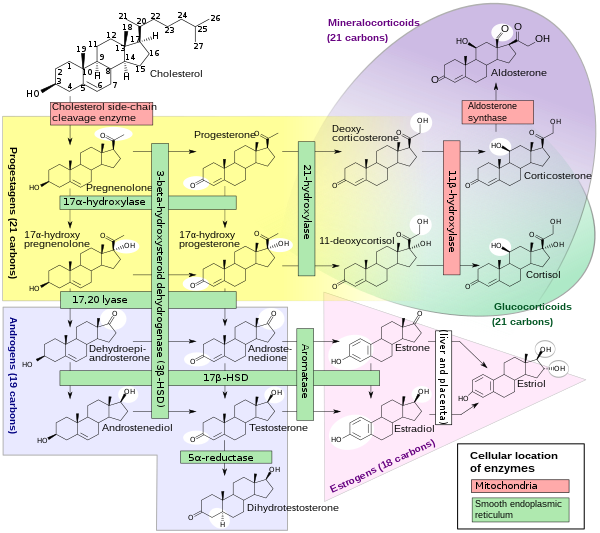 |
Additional images
[edit]-
Steroid numbering
See also
[edit]References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000148795 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000003555 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c d "CYP17A1 cytochrome P450 family 17 subfamily A member 1 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Archived from the original on 2015-06-23. Retrieved 2016-09-27.
- ^ "BioGPS - your Gene Portal System". biogps.org. Archived from the original on 2011-08-20. Retrieved 2016-10-11.
- ^ a b Boulpaep EL, Boron, WF (2005). Medical physiology: a cellular and molecular approach. St. Louis, Mo: Elsevier Saunders. p. 1180. ISBN 1-4160-2328-3.
- ^ a b Vasaitis TS, Bruno RD, Njar VC (May 2011). "CYP17 inhibitors for prostate cancer therapy". The Journal of Steroid Biochemistry and Molecular Biology. 125 (1–2): 23–31. doi:10.1016/j.jsbmb.2010.11.005. PMC 3047603. PMID 21092758.
- ^ a b Mega JL, Stitziel NO, Smith JG, Chasman DI, Caulfield M, Devlin JJ, et al. (June 2015). "Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials". Lancet. 385 (9984): 2264–2271. doi:10.1016/S0140-6736(14)61730-X. PMC 4608367. PMID 25748612.
- ^ "CYP17A1 - Steroid 17-alpha-hydroxylase/17,20 lyase - Homo sapiens (Human) - CYP17A1 gene & protein". www.uniprot.org. Archived from the original on 2016-10-12. Retrieved 2016-10-11.
- ^ Estrada DF, Laurence JS, Scott EE (February 2016). "Cytochrome P450 17A1 Interactions with the FMN Domain of Its Reductase as Characterized by NMR". The Journal of Biological Chemistry. 291 (8): 3990–4003. doi:10.1074/jbc.M115.677294. PMC 4759177. PMID 26719338.
- ^ DeVore NM, Scott EE (January 2012). "Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001". Nature. 482 (7383): 116–119. Bibcode:2012Natur.482..116D. doi:10.1038/nature10743. PMC 3271139. PMID 22266943.
- ^ Petrunak EM, DeVore NM, Porubsky PR, Scott EE (November 2014). "Structures of human steroidogenic cytochrome P450 17A1 with substrates". The Journal of Biological Chemistry. 289 (47): 32952–32964. doi:10.1074/jbc.M114.610998. PMC 4239641. PMID 25301938.
- ^ a b c Storbeck KH, Swart P, Africander D, Conradie R, Louw R, Swart AC (April 2011). "16α-hydroxyprogesterone: origin, biosynthesis and receptor interaction". Molecular and Cellular Endocrinology. 336 (1–2): 92–101. doi:10.1016/j.mce.2010.11.016. PMID 21095220. S2CID 5503049.
- ^ DeVore NM, Scott EE (January 2012). "Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001". Nature. 482 (7383): 116–119. Bibcode:2012Natur.482..116D. doi:10.1038/nature10743. PMC 3271139. PMID 22266943.
- ^ Udhane SS, Dick B, Hu Q, Hartmann RW, Pandey AV (September 2016). "Specificity of anti-prostate cancer CYP17A1 inhibitors on androgen biosynthesis". Biochemical and Biophysical Research Communications. 477 (4): 1005–1010. doi:10.1016/j.bbrc.2016.07.019. PMID 27395338.
- ^ Pandey AV, Miller WL (April 2005). "Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17". The Journal of Biological Chemistry. 280 (14): 13265–13271. doi:10.1074/jbc.M414673200. PMID 15687493.
- ^ a b Zhang LH, Rodriguez H, Ohno S, Miller WL (November 1995). "Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome". Proceedings of the National Academy of Sciences of the United States of America. 92 (23): 10619–10623. Bibcode:1995PNAS...9210619Z. doi:10.1073/pnas.92.23.10619. PMC 40663. PMID 7479852.
- ^ Fukami M, Homma K, Hasegawa T, Ogata T (April 2013). "Backdoor pathway for dihydrotestosterone biosynthesis: implications for normal and abnormal human sex development". Developmental Dynamics. 242 (4): 320–329. doi:10.1002/dvdy.23892. PMID 23073980. S2CID 44702659.
- ^ Masiutin M, Yadav M (2023). "Alternative androgen pathways". WikiJournal of Medicine. 10: X. doi:10.15347/WJM/2023.003. S2CID 257943362.
- ^ "Entrez Gene: CYP17A1 cytochrome P450, family 17, subfamily A, polypeptide 1". Archived from the original on 2010-03-07. Retrieved 2017-08-30.
- ^ Ma YN, Cao CY, Wang QW, Gui WJ, Zhu GN (October 2016). "Effects of azocyclotin on gene transcription and steroid metabolome of hypothalamic-pituitary-gonad axis, and their consequences on reproduction in zebrafish (Danio rerio)". Aquatic Toxicology. 179: 55–64. doi:10.1016/j.aquatox.2016.08.006. PMID 27571716.
- ^ Legendre A, Elie C, Ramambason C, Manens L, Souidi M, Froment P, et al. (August 2016). "Endocrine effects of lifelong exposure to low-dose depleted uranium on testicular functions in adult rat" (PDF). Toxicology. 368–369: 58–68. Bibcode:2016Toxgy.368...58L. doi:10.1016/j.tox.2016.08.014. PMID 27544493. Archived (PDF) from the original on 2024-02-02. Retrieved 2024-02-02.
- ^ Yadav R, Petrunak EM, Estrada DF, Scott EE (February 2017). "Structural insights into the function of steroidogenic cytochrome P450 17A1". Molecular and Cellular Endocrinology. 441: 68–75. doi:10.1016/j.mce.2016.08.035. PMC 5235955. PMID 27566228.
- ^ Kostin VA, Zolottsev VA, Kuzikov AV, Masamrekh RA, Shumyantseva VV, Veselovsky AV, et al. (November 2016). "Oxazolinyl derivatives of [17(20)E]-21-norpregnene differing in the structure of A and B rings. Facile synthesis and inhibition of CYP17A1 catalytic activity". Steroids. 115: 114–122. doi:10.1016/j.steroids.2016.06.002. PMID 27505042. S2CID 205256638.
- ^ a b Bonomo S, Hansen CH, Petrunak EM, Scott EE, Styrishave B, Jørgensen FS, et al. (July 2016). "Promising Tools in Prostate Cancer Research: Selective Non-Steroidal Cytochrome P450 17A1 Inhibitors". Scientific Reports. 6: 29468. Bibcode:2016NatSR...629468B. doi:10.1038/srep29468. PMC 4942611. PMID 27406023.
- ^ Acién P, Acién M (November 2020). "Disorders of Sex Development: Classification, Review, and Impact on Fertility". Journal of Clinical Medicine. 9 (11): 3555. doi:10.3390/jcm9113555. PMC 7694247. PMID 33158283.
- ^ Bordeau BM, Ciulla DA, Callahan BP (September 2016). "Hedgehog Proteins Consume Steroidal CYP17A1 Antagonists: Potential Therapeutic Significance in Advanced Prostate Cancer". ChemMedChem. 11 (18): 1983–1986. doi:10.1002/cmdc.201600238. PMC 5588864. PMID 27435344.
- ^ Fernández-Cancio M, Camats N, Flück CE, Zalewski A, Dick B, Frey BM, et al. (April 2018). "Mechanism of the Dual Activities of Human CYP17A1 and Binding to Anti-Prostate Cancer Drug Abiraterone Revealed by a Novel V366M Mutation Causing 17,20 Lyase Deficiency". Pharmaceuticals. 11 (2): 37. doi:10.3390/ph11020037. PMC 6027421. PMID 29710837.
- ^ PDB: 3ruk; DeVore NM, Scott EE (January 2012). "Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001". Nature. 482 (7383): 116–119. Bibcode:2012Natur.482..116D. doi:10.1038/nature10743. PMC 3271139. PMID 22266943.
- ^ "Tokai Pharmaceuticals' Reformulated Galeterone Demonstrates Robust PSA Reductions in Advanced Prostate Cancer Patients" (Press release). Tokai Pharmaceuticals. January 29, 2014.[permanent dead link]
- ^ Bird IM, Abbott DH (October 2016). "The hunt for a selective 17,20 lyase inhibitor; learning lessons from nature". The Journal of Steroid Biochemistry and Molecular Biology. 163: 136–146. doi:10.1016/j.jsbmb.2016.04.021. PMC 5046225. PMID 27154414.
VT464 is another recently developed compound proposed to act as a selective lyase inhibitor, and more complete data is available in the public domain to support this claim. A review of preliminary data released suggest the IC50 for Human CYP17 lyase activity is ten times lower than for hydroxylase 15 and in nonhuman primates VT464 was able to suppress circulating testosterone as effectively as abiraterone, but with minimally depressed cortisol (remaining at 82% control compared to only 9% with aberaterone), and without associated increases in pregnenolone, progesterone and mineralocorticoids otherwise observed with abiraterone. Like Galaterone, VT464 is also in use in clinical trials without co-administration of prednisone. Together with the clear lack of suppression of circulating cortisol in nonhuman primates, these data argue that VT464 may indeed be a selective 17,20 lyase inhibitor.
- ^ Madan RA, Schmidt KT, Karzai F, Peer CJ, Cordes LM, Chau CH, et al. (August 2020). "Phase 2 Study of Seviteronel (INO-464) in Patients With Metastatic Castration-Resistant Prostate Cancer After Enzalutamide Treatment". Clinical Genitourinary Cancer. 18 (4): 258–267.e1. doi:10.1016/j.clgc.2019.11.002. PMC 7415516. PMID 32327394.
- ^ Peer CJ, Schmidt KT, Kindrick JD, Eisner JR, Brown VV, Baskin-Bey E, et al. (October 2019). "A population pharmacokinetic analysis of the oral CYP17 lyase and androgen receptor inhibitor seviteronel in patients with advanced/metastatic castration-resistant prostate cancer or breast cancer". Cancer Chemotherapy and Pharmacology. 84 (4): 759–770. doi:10.1007/s00280-019-03908-0. PMC 8132106. PMID 31367790. S2CID 199056344.
Further reading
[edit]- Miura K, Yasuda K, Yanase T, Yamakita N, Sasano H, Nawata H, et al. (October 1996). "Mutation of cytochrome P-45017 alpha gene (CYP17) in a Japanese patient previously reported as having glucocorticoid-responsive hyperaldosteronism: with a review of Japanese patients with mutations of CYP17". The Journal of Clinical Endocrinology and Metabolism. 81 (10): 3797–3801. doi:10.1210/jcem.81.10.8855840. PMID 8855840.
- Miller WL, Geller DH, Auchus RJ (1999). "The molecular basis of isolated 17,20 lyase deficiency". Endocrine Research. 24 (3–4): 817–825. doi:10.3109/07435809809032692. PMID 9888582.
- Strauss JF (November 2003). "Some new thoughts on the pathophysiology and genetics of polycystic ovary syndrome". Annals of the New York Academy of Sciences. 997 (1): 42–48. Bibcode:2003NYASA.997...42S. doi:10.1196/annals.1290.005. PMID 14644808. S2CID 23559461.
- Haider SM, Patel JS, Poojari CS, Neidle S (July 2010). "Molecular modeling on inhibitor complexes and active-site dynamics of cytochrome P450 C17, a target for prostate cancer therapy". Journal of Molecular Biology. 400 (5): 1078–1098. doi:10.1016/j.jmb.2010.05.069. PMID 20595043.
External links
[edit]- CYP17A1+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Human CYP17A1 genome location and CYP17A1 gene details page in the UCSC Genome Browser.