User:JWB
American expansion and division period
[edit]Republic of Texas Texas Annexation Wilmot Proviso

Mexican-American War Treaty of Guadalupe Hidalgo Mexican Cession
U.S. provisional government of New Mexico Territorial evolution of New Mexico

Constitutional Convention (California)

Compromise of 1850 Gadsden Purchase

Other geography
[edit]


Language
[edit]Comparative linguistics
[edit]Linguistic typology Eurasiatic languages Borean languages
Writing systems
[edit]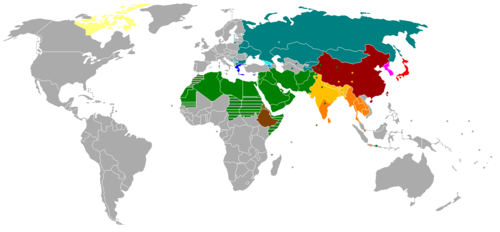
| Alphabet Latin Cyrillic&Latin Greek Georgian Armenian | Logographic+Syllabic Hanzi (L) Kana (2S)+Kanji(L) Hangul(Featural-alphabetic S)+limited Hanja(L) |
| Abjad Arabic&Latin Hebrew | Abugida N, S Indic Ethiopic Thaana Canadian Syllabic |
Writing system Determinative Radical (Chinese character) South_Arabian_alphabet#Sign_inventory History of the Arabic alphabet
East Asia
[edit]Standard Chinese Varieties of Chinese Mandarin Chinese Mandarin dialects Xiang Chinese Ported more detailed dialect map from Chinese Wikipedia Yue Chinese Tuhua
Anthropology
[edit]
Multiregional origin of modern humans Wallace Line Wallacea
Astronomy
[edit]Late Heavy Bombardment Nice model
Mathematics
[edit]
Nuclear
[edit]Arms control
[edit]List of states with nuclear weapons Nuclear-Weapon-Free Zone



Reactor technology
[edit]Types
[edit]Fast-neutron reactor Integral Fast Reactor UHTREX
Components
[edit]Nuclear reactor coolant Neutron moderator
Fission products
[edit]Fission product Fission products (by element) Fission product yield Fluoride volatility
| Nuclide | t1⁄2 | Yield | Q[a 1] | βγ |
|---|---|---|---|---|
| (Ma) | (%)[a 2] | (keV) | ||
| 99Tc | 0.211 | 6.1385 | 294 | β |
| 126Sn | 0.230 | 0.1084 | 4050[a 3] | βγ |
| 79Se | 0.327 | 0.0447 | 151 | β |
| 135Cs | 1.33 | 6.9110[a 4] | 269 | β |
| 93Zr | 1.53 | 5.4575 | 91 | βγ |
| 107Pd | 6.5 | 1.2499 | 33 | β |
| 129I | 16.14 | 0.8410 | 194 | βγ |
| t½ (year) |
Yield (%) |
Q (keV) |
βγ | |
|---|---|---|---|---|
| 155Eu | 4.76 | 0.0803 | 252 | βγ |
| 85Kr | 10.76 | 0.2180 | 687 | βγ |
| 113mCd | 14.1 | 0.0008 | 316 | β |
| 90Sr | 28.9 | 4.505 | 2826 | β |
| 137Cs | 30.23 | 6.337 | 1176 | βγ |
| 121mSn | 43.9 | 0.00005 | 390 | βγ |
| 151Sm | 88.8 | 0.5314 | 77 | β |
| Actinides[1] by decay chain | Half-life range (a) |
Fission products of 235U by yield[2] | ||||||
|---|---|---|---|---|---|---|---|---|
| 4n | 4n + 1 | 4n + 2 | 4n + 3 | 4.5–7% | 0.04–1.25% | <0.001% | ||
| 228Ra№ | 4–6 a | 155Euþ | ||||||
| 248Bk[3] | > 9 a | |||||||
| 244Cmƒ | 241Puƒ | 250Cf | 227Ac№ | 10–29 a | 90Sr | 85Kr | 113mCdþ | |
| 232Uƒ | 238Puƒ | 243Cmƒ | 29–97 a | 137Cs | 151Smþ | 121mSn | ||
| 249Cfƒ | 242mAmƒ | 141–351 a |
No fission products have a half-life | |||||
| 241Amƒ | 251Cfƒ[4] | 430–900 a | ||||||
| 226Ra№ | 247Bk | 1.3–1.6 ka | ||||||
| 240Pu | 229Th | 246Cmƒ | 243Amƒ | 4.7–7.4 ka | ||||
| 245Cmƒ | 250Cm | 8.3–8.5 ka | ||||||
| 239Puƒ | 24.1 ka | |||||||
| 230Th№ | 231Pa№ | 32–76 ka | ||||||
| 236Npƒ | 233Uƒ | 234U№ | 150–250 ka | 99Tc₡ | 126Sn | |||
| 248Cm | 242Pu | 327–375 ka | 79Se₡ | |||||
| 1.33 Ma | 135Cs₡ | |||||||
| 237Npƒ | 1.61–6.5 Ma | 93Zr | 107Pd | |||||
| 236U | 247Cmƒ | 15–24 Ma | 129I₡ | |||||
| 244Pu | 80 Ma |
... nor beyond 15.7 Ma[5] | ||||||
| 232Th№ | 238U№ | 235Uƒ№ | 0.7–14.1 Ga | |||||
| ||||||||
Actinides
[edit]| 237Np | ||||||||||||||
| ↑ | ||||||||||||||
| 231U | ← | 232U | ↔ | 233U | ↔ | 234U | ↔ | 235U | ↔ | 236U | → | 237U | ||
| ↓ | ↑ | ↑ | ↑ | |||||||||||
| 231Pa | → | 232Pa | ← | 233Pa | → | 234Pa | ||||||||
| ↑ | ↑ | |||||||||||||
| 230Th | → | 231Th | ← | 232Th | → | 233Th | ||||||||
| ||||||||||||||

Isotopes
[edit]Table of nuclides Isotope Tritium
- ^ Plus radium (element 88). While actually a sub-actinide, it immediately precedes actinium (89) and follows a three-element gap of instability after polonium (84) where no nuclides have half-lives of at least four years (the longest-lived nuclide in the gap is radon-222 with a half life of less than four days). Radium's longest lived isotope, at 1,600 years, thus merits the element's inclusion here.
- ^ Specifically from thermal neutron fission of uranium-235, e.g. in a typical nuclear reactor.
- ^ Milsted, J.; Friedman, A. M.; Stevens, C. M. (1965). "The alpha half-life of berkelium-247; a new long-lived isomer of berkelium-248". Nuclear Physics. 71 (2): 299. Bibcode:1965NucPh..71..299M. doi:10.1016/0029-5582(65)90719-4.
"The isotopic analyses disclosed a species of mass 248 in constant abundance in three samples analysed over a period of about 10 months. This was ascribed to an isomer of Bk248 with a half-life greater than 9 [years]. No growth of Cf248 was detected, and a lower limit for the β− half-life can be set at about 104 [years]. No alpha activity attributable to the new isomer has been detected; the alpha half-life is probably greater than 300 [years]." - ^ This is the heaviest nuclide with a half-life of at least four years before the "sea of instability".
- ^ Excluding those "classically stable" nuclides with half-lives significantly in excess of 232Th; e.g., while 113mCd has a half-life of only fourteen years, that of 113Cd is eight quadrillion years.
